Water is healthy, even vital, but can also be toxic in large amounts. We do not have to worry about becoming dehydrated under normal circumstances, if we drink when we’re thirsty and stop when we’re not. It can be dangerous if we force ourselves to drink exorbitant amounts of water and don’t listen to our bodies. In this part, we have a look at treating water poisoning and at an effective biochemical control system in our bodies which takes care of our optimal daily water intake.
5. What Does One Do in the Case of Water Poisoning?
Thanks to a truly special coincidence, we can compare two different contemporary therapeutic approaches to the problem, with both of the patients being trained physicians. In October of 1983, the American Medical Joggers Association (!), at its annual meeting in Chicago, arranged for ultramarathon events covering 80 and 100 km.
A 24-year-old medical student, R. Tyler Frizzell, and a 45-year-old physician, Dr. S. Robert Lanthan, both experienced marathon and ultramarathon runners, participated in the race (see Tab. 2). In 1986, these two reported on various external circumstances and all the medically relevant details of their cases from this, for them, dramatic race in the Journal of the American Medical Association, the general medicine publication with the world’s widest circulation [33].
Before the race, all participants were encouraged to drink 300–360 mL of liquid at each of the resupply stations, located at intervals of 1.6 km. This corresponded to recommendations from the American Medical Joggers Association, and was also consistent with the then current state of medical knowledge. It was further suggested that “they drink more than their thirst demanded” since thirst during a race was regarded as an unreliable indicator of one’s actual liquid requirement. Furthermore, participants were advised to “force themselves to consume liquids”.
Following this advice, and throughout the race in question—which was taking place at 90 °F, or 32 °C—each of the two subjects in question consumed more than 20 liters of liquid. Despite their different ages, distances traveled, and run times, both runners were in pathetic condition when the race ended: listless, disoriented, confused, and responsive only in a limited way. Each was immediately admitted to the emergency room, where they were routinely subjected to quite a number of diagnostically relevant tests (see Tab. 2).
|
Table 2. Clinical data of two participants in ultramarathons. |
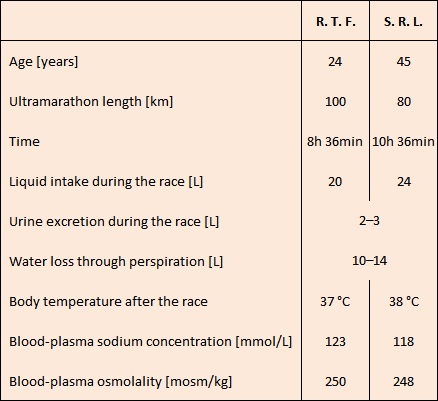 |
On the basis of their body temperatures, which were normal, heat stroke could be ruled out in both cases. A combination of low osmolalities (normal value: 290 mosm/kg) as well as low sodium concentrations (normal value: 144 mmol/L) pointed clearly toward water poisoning. Hours of sweating had contributed to their low sodium values. The two athletes arrived at the emergency room at different times, which is apparently why they were treated differently.
Over the course of eight hours, S. R. L. was given a total of 2.1 L of 3 % hypertonic salt solution intravenously. After the first three hours, the patient was already reacting completely normally. His sodium value climbed from a menacing 118 mmol/L to over 135 mmol/L, and he discharged over 4 liters of urine. S. R. L. was subsequently discharged and was able to resume his normal medical practice that same afternoon.
R. T. F., however, was less fortunate. He was instead administered intravenously an 0.9 % (i.e., isotonic) salt solution. He was barely responsive, and suffered an epileptic attack, with temporary respiratory arrest. He remained for 36 hours in a semi-coma, during which time his sodium values rose only very slowly. The patient spent five days in the hospital before he could be discharged.
This report offers impressive evidence of what is therapeutically sensible after a water poisoning. Only through the immediate administration of salt, either in solid form or intravenously as a hypertonic solution (>2 %), can the extracellular osmotic pressure be rapidly diminished. Water then passes from intra- to extracellular regions, and swelling of the brain subsides. Once this is achieved, the kidneys discharge the excess water in the form of urine within the next few hours. On the other hand, oral or intravenous delivery of isotonic—or, worse yet, hypotonic—fluids (e.g., water) in the case of water poisoning is not only senseless but indeed life-threatening.
6. Aquaporins—Water channels through the cell membrane
How do water molecules even manage to pass through a cell membrane? Given their completely non-polar and hydrophobic interior, cell membranes present an effective barrier to molecules of any sort. In fact, for charged particles like H+, Na+, Mg2+, as well as large molecules like proteins, they are essentially impenetrable. Water, too, despite its small size, can permeate only to a limited extent through an artificial membrane. It long remained a mystery why certain natural membranes—for example, those surrounding red blood cells—are almost totally water-permeable [34].
A first experimental indication of the existence of selective “water channels” was unveiled by Robert Macey in 1970 [35]. Through the addition of mercury ions, he was able to drastically reduce the water permeability of human red blood cells. Apparently, Hg2+ ions blocked the entrances to these water channels in some unknown fashion.
It was not until more than 25 years later that Peter Agre [36,37] managed to identify and isolate the water channels in red blood cells: a protein that he called “aquaporin”. He was honored “for the discovery of water channels” with the 2003 Nobel Prize in Chemistry [38,39]. It turned out that Agre’s aquaporin was only the first member of what proved to be a whole group of water channels, and it was thus later redesignated as “aquaporin-1”.
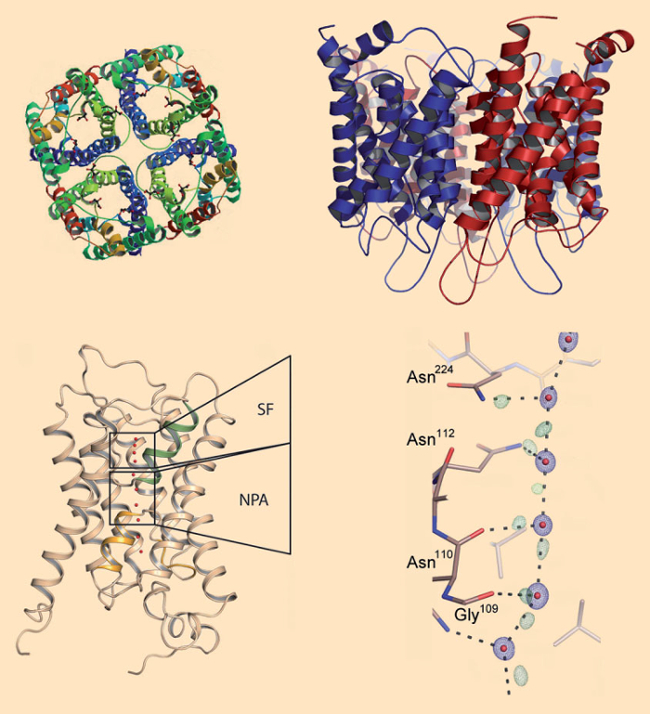
Aquaporins are present in all life forms, from bacteria, through plants, all the way to humans. Indeed, our bodies contain at least 13 different aquaporins. All aquaporins share a similar funnel-shaped constitution (see. Fig. 3). Four identical subunits come together in a quaternary structure spanning the cell membrane. Through each subunit, there is a central canal with a bottleneck only 0.3 nm wide, which forces water molecules to pass in single file but at the almost unbelievable rate of roughly 4 billion (!) water molecules per second!
 |
|
Figure 3. Aquaporins. a,b) Aquaporins consist of four identical protein subunits, each of which traverses the membrane with six helices. c) The selection filter (SF) of the channel includes the narrowest section, with a diameter of only 0.3 nm: roughly the size of a water molecule. In the so-called NPA section at the middle of the channel, water molecules rotate by roughly 180° during their transit. d) From ultraprecise X-ray structural data, the positions of a few hydrogen bonds between water molecules and amino acid side-chains can be determined. This produces a precise picture of how water molecules inside the channels are transported further [40]. |
It required high-precision X-ray structure analyses to provide a more detailed insight into the processes associated with transport of water molecules through such a channel. Figures. 3c and 3d illustrate the aquaporin structure with a resolution of 0.088 nm [40]. On the basis of this precise data, refined calculations allow the localization of a portion of the hydrogen bonds extending out from the water molecules. It becomes apparent that water molecules proceed through such a protein channel like a column of molecular canal diggers. Each water molecule is secured to the preceding and following water molecules by means of a hydrogen bond.
Along the walls of the channels are two types of hooks: proton donors that can bind to the oxygen of a water molecule, as well as proton acceptors which can bind one hydrogen atom of a water molecule. In the walls of the channels, the amino-acid side chains of the aquaporin proteins are precisely arranged so that the water molecules can make their way “hand-over-hand” through the entire channel.
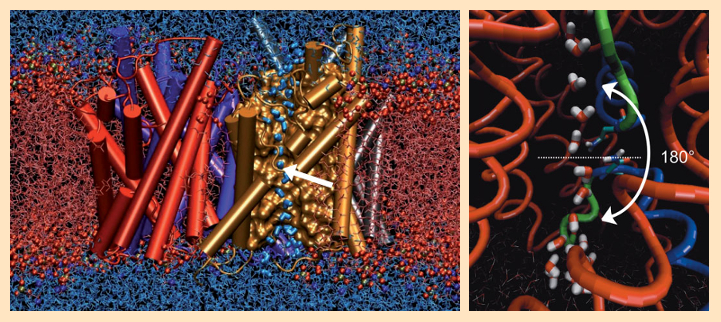
Extensive molecular dynamics calculations brought to light astonishing further results. In the middle of the membrane, there are two identical opposing NPA (asparagine-proline-alanine) sequences between two protein helices. Here, each passing water molecule executes a rotation of approximately 180° (see Fig. 4). As a result of this rotation, the hydrogen-bond connectivity along the H2O chain is necessarily interrupted, which hinders rapid Grothuss diffusion of protons through the membrane [41–43].
 |
|
Figure 4. Molecular dynamics calculations of water flow through an aquaporin. |
The chemistry of this seemingly simple water channel points not only to a fascinating bit of basic scientific research, but affects each one of us directly. Should you wish to experience a bit of your own aquaporin-2 system in action, the following two-day self-experiment offers a convenient opportunity.
7. Aquaporin Self-Experiments
Day 1: Aquaporin-2 in Action
Imagine you go to bed one evening, and sleep through until morning. There is, of course, nothing really special about that, but only because your aquaporin-2 system was active. As you slept, your pituitary gland released increased amounts of the antidiuretic hormone (ADH) into your bloodstream. This led in turn to increased release of aquaporin-2 into the outermost layer of cells in the collecting duct systems of both of your kidneys. Water is thus reabsorbed there to a greater extent, so that urine in the collecting ducts becomes more highly concentrated, and thus you do not need to go to the restroom during the night.
It is all actually even more complicated: ADH binds to and stimulates the so-called V2 receptors on the outer layer of cells of the collecting ducts of both your kidneys. This, in turn, triggers a reaction cascade leading to phosphorylation of serine sidechains 256, 261, 264, and 269 in aquaporin-2. As a result of this reaction, aquaporin previously stored in vesicles is set free and subsequently incorporated into the cell membrane [44].
Day 2: Aquaporin-2 Calm at First, Then Active
In the evening, assume you drink two glasses of wine. Soon you are conscious of an ever-increasing urge to urinate.
It’s no wonder: you’ve consumed half a liter of water. Worse yet, the alcohol present in the wine inhibits the release of ADH. This, in turn, leads to a diminished release of aquaporin-2, as a result of which less water is absorbed back from urine into the kidneys’ collecting ducts. The result: you need to dispose of large amounts of dilute, nearly colorless urine, perhaps causing you to frequently go to the restroom. The volume of urine released proves to be substantially greater than the sum of the two consumed glasses of wine.
During the night, after degradation of the alcohol, more ADH will again be released, and you can then sleep through the night. The next morning, you awake slightly dehydrated and thirsty, but still with a high level of ADH in your blood. Even though you proceed once again to drink liquids, urine in the collecting ducts again becomes more concentrated, and you don’t need to go to the restroom. The first time in the course of the following morning that you DO go, you’ll be pleased to discover that the urine is significantly darker, which proves how busy your aquaporin-2 has been at work.
It’s always surprising the way that chemistry turns out to be behind even the simplest everyday things. Who would have thought how much surprising, remarkable chemistry is hidden behind a single glass of water! We should, therefore, enjoy every sip and celebrate both osmotic pressure equalization and our Nobel-Prize-winning water channels.
References
[33] R. T. Frizzell et al., Hyponatremia and Ultramarathon Running, J. Am. Med. Assoc. 1986, 255, 772. https://doi.org/10.1001/jama.1986.03370060086025
[34] A. Skerra, Wie fließt Wasser durch die biologische Membran? (in German), Nachr. Chem. 2002, 50, 491. https://doi.org/10.1002/nadc.20020500419
[35] R. I. Macey et al., Inhibition of water and solute permeability in human red cells, Biochim. Biophys. Acta 1970, 211, 104. https://doi.org/10.1016/0005-2736(70)90130-6
[36] B. M. Denker et al., Identification, Purification, and Partial Characterization of a Novel Mr 28,000 Integral Membrane Protein from Erythrocytes and Renal Tubules, J. Biol.Chem. 1988, 263, 15634.
[37] P. Agre, The Aquaporin Water Channels, Proc. Am. Thorac. Soc. 2006, 3, 5. https://doi.org/10.1513/pats.200510-109JH
[38] P. Agre, Aquaporin Water Channels (Nobel Lecture), Angew. Chem. Int. Ed. 2004, 20, 4278. https://doi.org/10.1002/anie.200460804
[39] Nobel Lecture by Peter Agre (Video), www.nobelprize.org, 2003.
[40] U. K. Eriksson et al., Subangstrom Resolution X-Ray Structure Details Aquaporin-Water Interactions, Science 2013, 340, 1346. https://doi.org/10.1126/science.1234306
[41] D. Marx, Protonenwanderung im virtuellen Labor (in German), Spektrum der Wissenschaft 1999, 7, 21.
[42] B. L. de Groot et al., Water Permeation Across Biological Membranes: Mechanism and Dynamics of Aquaporin-1 and GlpF, Science 2001, 294, 2353. https://doi.org/10.1126/science.1066115
[43] E. Tajkhorshid et al., Control of the Selectivity of the Aquaporin Water Channel Family by Global Orientational Tuning, Science 2002, 296, 525. https://doi.org/10.1126/science.1067778
[44] H. B. Moeller et al., Phosphorylation of aquaporin-2 regulates its endocytosis and protein–protein interactions, Proc. Natl. Acad. Sci. USA 2010,107, 424.
The article has been published in German as:
- Dehydrierung: Die Angst geht um,
Klaus Roth,
Chem. Unserer Zeit 2014, 48, 332–340.
DOI: 10.1002/ciuz.201400688
and was translated by W. E. Russey.
Can Too Much Water Be Toxic? – Part 1
Is such a thing as “water poisoning” possible?
Can Too Much Water Be Toxic? – Part 2
How can water cross cell membranes? Fascinating chemistry that affects each one of us
See all articles by Klaus Roth




