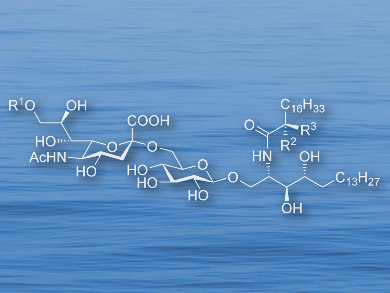
Sialic acids are the vital component for the biological activity of gangliosides (lipids found in cell membranes). However, the stereoselective addition of sialic acid derivatives to glycosyl moieties remains challenging. Hence, an efficient method for α-selective sialylation would be useful.
Shun-Yuan Luo, National Chung Hsing University, Taichung, Taiwan, and colleagues have developed a protocol for creating α-sialosides in high yields. The method is based on a pre-activated 5-N,4-O-carbamate thiosialoside donor and uses p-toluenesulfenyl chloride (p-TolSCl) and silver triflate (AgOTf) to promote the reaction. The approach was applied to the total synthesis of the gangliosides Hp-s1, DSG-A, and their analogues.
The strategy is mild and was further expanded to work with a range of sugars. A mechanism for the pre-activation reaction was proposed according to physical and chemical data analysis. The final compound shoued an enhanced neuritogenic activity on SH-SY5Y cells, meaning that these compounds show promise in the treatment of neuronal injuries and/or neurodegenerative diseases.
- Mild and Highly α-Selective O-Sialylation Method Based on Pre-Activation: Access to Gangliosides Hp-s1, DSG-A, and Their Analogues,
Ganesh B. Shelke, Bo-Rong Chen, Shih-An Yang, Tzer-Min Kuo, Yong-Long Syu, Ying-Chin Ko, Shun-Yuan Luo,
Asian J. Org. Chem. 2017.
DOI: 10.1002/ajoc.201700358