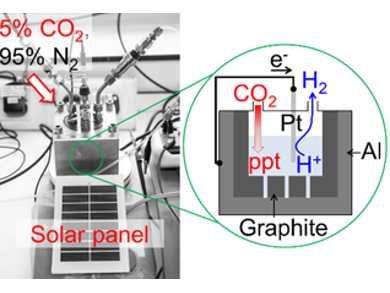
Pedro M. Aguiar, Michael North, Alison Parkin, and colleagues, University of York, UK, have developed an electrochemical cell for CO2 mineralization. The cell consists of a novel dual-component graphite and Earth-crust abundant metal anode, a hydrogen producing cathode (Pt, Ni, or Fe), and an aqueous sodium chloride electrolyte. Carbon capture can be achieved in a highly sustainable manner using scrap metal within the anode (e.g., waste aluminium foil), seawater as the electrolyte, an industrially relevant gas stream, and a solar panel as an effective zero-carbon energy source.
Under an atmosphere of 5 % CO2 in nitrogen, the cell showed capacitive and oxidative electrochemistry at the anode. The graphite acted as a supercapacitive reagent concentrator, pumping CO2 into the aqueous solution as hydrogen carbonate. Simultaneous oxidation of the anodic metal generated cations. These reacted with the hydrogen carbonate to give mineralized CO2 (e.g., an insoluble aluminium hydroxycarbonate mineral). Whilst conventional electrochemical CO2 reduction requires hydrogen, this cell generates hydrogen at the cathode.
According to the researchers, it is 33 % more energy efficient to use waste aluminium this way rather than to recycle it. Future work will focus on optimizing the cell design and to investigate the effect of impurities in the CO2 gas stream.
- Capacitance-Assisted Sustainable Electrochemical Carbon Dioxide Mineralisation,
Katie J. Lamb, Mark R. Dowsett, Konstantinos Chatzipanagis, Zhan Wei Scullion, Roland Kröger, James D. Lee, Pedro M. Aguiar, Michael North, Alison Parkin,
ChemSusChem 2018.
https://doi.org/10.1002/cssc.201702087