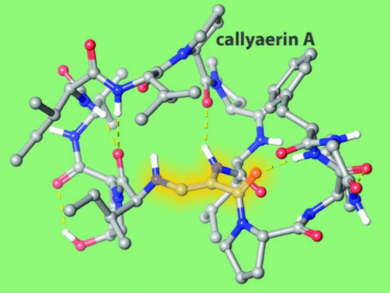
Tuberculosis (TB), a highly contagious and airborne disease caused by Mycobacterium tuberculosis, is a leading cause of mortality worldwide. Callyaerin A is a cyclic peptide derived from the Indonesian marine sponge Callyspongia aerizusa. It features a rare (Z)-2,3-diaminoacrylamide (DAA) moiety and is a potent inhibitor of M. tuberculosis with no cytotoxicity observed in human cells.
Margaret A. Brimble, University of Auckland, New Zealand, and colleagues have successfully executed the first synthesis of callyaerin A. The solid phase synthesis of the linear precursor to callyaerin A uses an Fmoc-protected formylglycine-diethylacetal as a masked form of formylglycine (FMoc = fluorenylmethyloxycarbonyl). An intramolecular cyclization between the formylglycine and the N-terminal amine of the linear peptide precursor was used to construct the rare DAA bridge.
Variable-temperature NMR studies of both callyaerin A and its all-amide analogue demonstrated the high conformational rigidity imposed by the DAA moiety on the cyclic peptide structure. The all-amide analogue was inactive against TB. This highlights the important role that the rare DAA moiety plays for bioactivity. Thus, the DAA group has potential as a peptide conformation constraining agent.
- Total Synthesis and Conformational Study of Callyaerin A: Anti-Tubercular Cyclic Peptide Bearing a Rare Rigidifying (Z)-2,3- Diaminoacrylamide Moiety,
Shengping Zhang, Luis M. De Leon Rodriguez, Ivanhoe K. H. Leung, Gregory M. Cook, Paul W. R. Harris, Margaret A. Brimble,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201712792