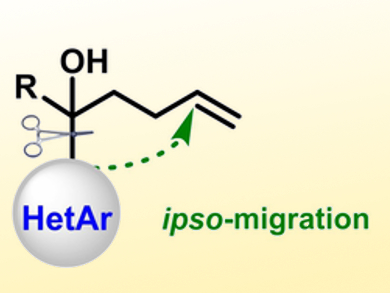
Organosilicon compounds are commonly used in materials science, energy development, and drug discovery. The radical-mediated silylation of alkenes provides a practical approach for production of organosilicon compounds. However, the silylation of unactivated alkenes is still very challenging.
Chen Zhu and colleagues, Soochow University, Suzhou, China, have developed a copper-catalyzed heteroarylsilylation of unactivated olefins via an intramolecular heteroaryl migration (pictured) which is triggered by the addition of silyl radicals to alkenes. The team used triphenylsilane as a silicon source, CuO as a catalyst, tert-butyl peroxyacetate to generate radicals, and 4-dimethylaminopyridine (DMAP) as a base.
The method can be used with a range of migrating heteroaryls, including benzothiazolyl, thiazolyl, imidazolyl, and pyridyl groups. The reactions proceed in synthetically useful yields, have good chemo- and regioselectivities, and tolerate a variety of functional groups. The approach allows the easy synthesis of heteroaryl-substituted alkyl silanes from readily available alkenes.
- Copper-Catalyzed Heteroarylsilylation of Unactivated Olefins through Distal Heteroaryl Migration,
Hong Zhang, Xinxin Wu, Qian Zhao, Chen Zhu,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201800150