Rechargeable aqueous metal-ion batteries (RAMBs) are a safe, low-cost, and environmentally friendly battery technology. However, RAMBs suffer from capacity fading because of H2/O2 evolution reactions, proton co-intercalation of ions contained within the electrolyte, and dissolution of electrode materials in an aqueous setting. Hybrid aqueous batteries (HABs) containing selective cation channels could extend the lifetime of RAMB electrodes.
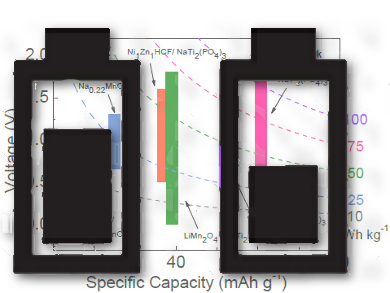
Mianqi Xue and Rui Li, Peking University, Shenzhen, China, and coworkers have designed a mixed potassium–sodium HAB containing a Prussian blue K2FeFe(CN)6 cathode (K-FeHCF) and a carbon-coated NaTi2(PO4)3 anode (NTP/C). The cathode and anode are selective for K+ and Na+ ions, respectively. Ultrafast ion conduction is possible with NTP/C.
A HAB battery containing the K-FeHCF and NTP/C electrodes delivers a 160 mAh g–1 capacity at 0.5 C. The researchers suggest that the battery’s 69.6 Wh kg–1 energy density is comparable to that of commercial mixed-ion aqueous batteries such as lead acid, Ni/Cd, and nickel—metal hydride batteries.
- Engineering Fast Ion Conduction and Selective Cation Channels for High-Rate and High-Voltage Hybrid Aqueous Battery,
Chunyi Liu, Xusheng Wang, Wenjun Deng, Chang Li, Jitao Chen, Mianqi Xue, Rui Li, Feng Pan,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201800479