Chelating ligands like EDTA (ethylenediaminetetraacetic acid) are widely used to enhance the preservation of consumer products as diverse as face cream and mayonnaise. They affect the uptake and usage of essential metal ions by microorganisms. However, the way in which they do this is not well understood. In additon, there is a need for “greener” alternatives to EDTA.
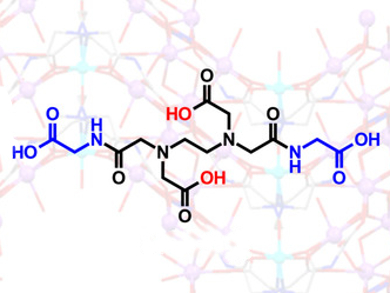
J. A. Gareth Williams, Gary J. Sharples, and Raminder S. Mulla, Durham University, UK, and colleagues have synthesized two symmetrical bis‐amide derivatives of EDTA and evaluated their ability to inhibit the growth of a variety of bacteria. The ligands are related to EDTA but feature amide substituents carrying glycyl (AmGly2) or pyridyl groups (AmPy2). AmGly2 is obtained from EDTA bis-anhydride and glycine, whereas AmPy2 is best prepared by initial protection of the amines.
AmGly2 (pictured) turns out to be a more potent inhibitor of Escherichia coli growth at lower concentrations than EDTA, even though its metal-binding affinities are lower for all common metal ions. E. coli mutants with defective outer-membrane structures were also studied to shed light on the behavior. The results indicate that metal ion binding characteristics are not necessarily a good predictor of bacterial growth inhibition of a given chelating ligand. In addition, EDTA bis‐amides necessitate further investigation as a new class of chelating antimicrobials.
- On the Antibacterial Activity of Azacarboxylate Ligands: Lowered Metal Ion Affinities for Bis-amide Derivatives of EDTA do not mean Reduced Activity,
Raminder S. Mulla, Marikka S. Beecroft, Robert Pal, Juan A. Aguilar, Javier Pitarch-Jarque, Enrique García-España, Elena Lurie-Luke, Gary J. Sharples, J. A. Gareth Williams,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201800026