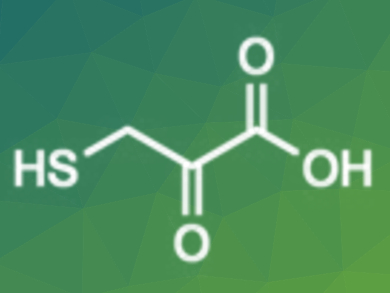
3-Mercaptopyruvate is a crucial sulfur metabolite. It is involved in various redox cellular processes and has therapeutic potential as an antidote to cyanide poisoning. However, the syntheses reported so far are tedious and do not lead to 3-mercaptopyruvatic acid (pictured), but to its dimer.
Erwan Galardon, CNRS, Université Paris Descartes, Sorbonne Paris Cité, France, and Jean-Christophe Lec, CNRS, Université de Lorraine, Nancy, France, have developed a simple synthesis of 3-mercaptopyruvatic acid with high purity. The team started from bromopyruvic acid in dichloromethane and added triphenylmethanethiol and N,N‐diisopropylethylamine to give a 3-mercaptopyruvatic acid derivative with a trityl-protected sulfur atom. Deprotection using trifluoroacetic acid and triisopropylsilane in dichloromethane gave the desired product in an overall yield of 54 %. The product was characterized using 1H and 13C NMR spectroscopy and elemental analysis.
The results indicate that the commercially available 3-mercaptopyruvate is generally not the monomer (CAS 10255-67-1), but its cyclic dimer disodium 2,5-di-hydroxy-1,4-dithiane-2,5-dicarboxylate (CAS 1309654-46-3/1001081-70-4). According to the researchers, dimer formation could influence the role of 3-mercaptopyruvate in biological systems.
- Synthesis, Characterisation and Reactivity of 3-Mercaptopyruvic Acid,
Erwan Galardon, Jean-Christophe Lec,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800199