The design of inorganic open‐framework materials is of interests because of their architectures/topologies, physical properties, and potential applications in various areas such as ion exchange, industrial catalysis, molecular separation, and proton‐conduction. Zeolites are, so far, the most representative example among these materials. Not much effort has been taken to explore the chemistry of metal‐halide‐based open frameworks. Lead‐halide perovskites (haloplumbates) are an important subclass of these materials which have raised research interests due to their potential applications in optoelectronic devices and to their fascinating structural diversity. However, the majority of reported haloplumbates are low-dimensional polymeric structures, i.e., clusters, ribbons, or 2D layers. Until now, the number of 3D haloplumbate frameworks has been very limited.
Qihua Xiong, Qichun Zhang, and colleagues, Nanyang Technological University, Singapore, have prepared a 3D haloplumbate framework, [H4TIMM]Pb7Br18 (TIMM = tetrakis(N‐imidazolemethylene)methane). The team reacted PbBr2, HBr, and TIMM under hydrothermal conditions and obtained the framework in a yield of 25 %.
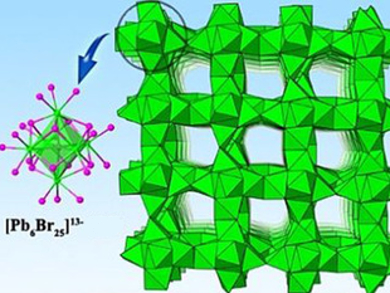
The framework was characterized using single‐crystal X‐ray diffraction. It is constructed from [Pb6Br25]13– nanoclusters (pictured left), which connect to each other through sharing faces and edges. H4TIMM acts as a template to direct the assembly of the [Pb6Br25]13– nanoclusters into an extended framework. The Pb2+ ions in the framework adopt seven‐ and eight-coordinated structures, which are very rare in haloplumbate chemistry. The synthesized framework is the first example of haloplumbates completely made from such highly‐coordinated Pb2+ ions.
- A 3D Haloplumbate Framework Constructed From Unprecedented Lindqvist-like Highly Coordinated [Pb6Br25]13– Nanoclusters with Temperature-Dependent Emission,
Xinxiong Li, T. Thu Ha Do, A. Granados del Águila, Yinjuan Huang, Wangqiao Chen, Qihua Xiong, Qichun Zhang,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201801292