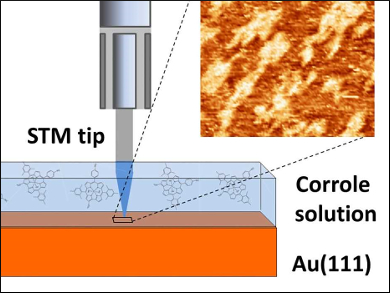
Corrole is an aromatic tetrapyrrole. Corrole derivatives have been used in many applications at the solid–liquid interface such as catalysis and sensing. In most of these applications, the controlled deposition of corrole layers is important, because this step is fundamental to tune the behavior of the macrocycle for the target function. Although the structural arrangement of the molecular layers in contact with the liquid is of fundamental interest, studies of such corrole layers have been rare.
Beatrice Bonanni, University of Rome “Tor Vergata”, Italy, and colleagues have investigated the deposition of the water‐soluble phosphorus complex of a 2‐sulfonato‐10‐(4‐sulfonatophenyl)‐5,15‐dimesitylcorrole onto an Au(111) surface. The team used scanning tunneling microscopy (STM) to monitor the layer formation in situ (pictured).
The researchers found that the corrole molecules arrange flat on gold. There were significant differences in the morphology of corrole layers formed in the presence of liquid and those deposited after solvent evaporation. At the solid–liquid interface, the compound forms plateau-like clusters with stable surface coverage in the range of 65–75 % at higher concentrations. When the solvent was evaporated (as for drop-casting deposition), the coating of gold was less effective and less stable during repetitive scans.
The team also verified that corrole molecules preserve their redox functionality after adsorption on the gold substrate. This is significant for possible applications of these corrole layers as surface coatings in solution, e.g., for the sanitization of water tanks.
- Corroles at the Real Solid-Liquid Interface: In Situ STM Investigation of a Water-Soluble Corrole Layer Deposited onto Au(111),
Beatrice Bonanni, Laura Fazi, Massimo Fanfoni, Anna Sgarlata, Fabrizio Caroleo, Giuseppe Pomarico, Pierluca Galloni, Federica Sabuzi, Luca Persichetti, Roberto Paolesse, Claudio Goletti,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201803802