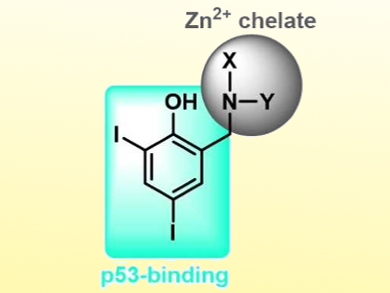
Over 50 % of cancer diagnoses are attributed to a loss of function in the p53 protein which functions as a tumor suppressor. Therefore, the pharmacological reactivation of p53 is a key target in cancer research. Mutations due to cancer often render this protein inactive due to local protein unfolding, aggregation, and a loss of structural zinc ions located within the protein’s core DNA-binding domain.
Christian Gaiddon, Université de Strasbourg, France, Jeffrey J. Warren, Tim Storr, Simon Fraser University, Burnaby, Canada, and colleagues have developed a series of multifunctional ligands that restore the original wild-type function to mutant p53. The ligand design (pictured) includes iodinated phenols (blue) to stabilize a mutant-induced cavity and inhibit protein unfolding, and metal chelators (grey) which bind and increase intracellular Zn2+ to repopulate the metal-depleted site.
Cytotoxicity assays showed that the molecules were highly cytotoxic on a wide range of cancer cell lines. The lead small molecule in this study selectively induced apoptosis (programmed cell death) in a mutant p53 cell line, reduced levels of unfolded mutant p53, and recovered transcriptional p53 function. Overall, according to the researchers, the multifunctional scaffolds have the potential to restore wild-type function to mutant p53, showing promise for cancer treatment.
- Multifunctional Compounds for Activation of the p53-Y220C Mutant in Cancer,
Jessica J. Miller, Christophe Orvain, Shireen Jozi, Ryan M. Clarke, Jason R. Smith, Anaïs Blanchet, Christian Gaiddon, Jeffrey J. Warren, Tim Storr,
Chem. Eur. J. 2018, 24, 17734–17742.
https://doi.org/10.1002/chem.201802677