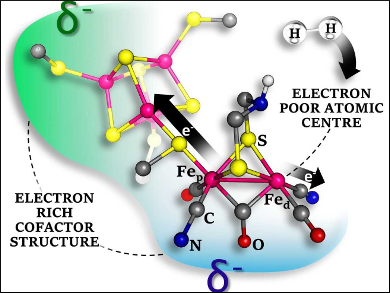
The reversible oxidation of H2 can be efficiently catalyzed by certain hydrogenase enzymes. The active site of the [FeFe]- variant, which contains iron ions together with CO, CN–, and S-based ligands, has attracted chemists’ interest. The aim has been to synthetically reproduce the natural function of the enzyme. However, though many bioinspired models are promising for H2 formation, only a few models with poor activity have been reported for the reverse H2 oxidation.
Luca De Giola, Giuseppe Zampella, and colleagues, University of Milano-Bicocca, Italy, have studied the mechanism of H2 binding/oxidation at the natural enzyme core compared with synthetic diiron dithiolate models. They used density functional theory (DFT) computations to simulate the complicated organometallic reactions and to understand why nature outperforms the synthetic models.
According to the results, the key to the success of the enzyme is the presence of a single electron-poor Fe atom, which favors H2 binding. Its surroundings are electron-rich, which supports H2 oxidation. CN– is the main contributor among the ligands to achieve that effect. This suggests that the use of CN− in the catalyst architecture of biomimetics should be considered.
- H2 Activation in [FeFe]-Hydrogenase Cofactor Versus Diiron Dithiolate Models: Factors Underlying the Catalytic Success of Nature and Implications for an Improved Biomimicry,
Federica Arrigoni, Luca Bertini, Maurizio Bruschi, Claudio Greco, Luca De Gioia, Giuseppe Zampella,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201804687