The octet rule for main-group elements and the 18-electron rule for transition metals are useful tools in modern chemistry to rationalize and predict the structure of molecules. They were suggested along with the 32-electron rule for the lanthanides and actinides almost 100 years ago, prior to modern quantum theory. These rules are valid for a large majority of stable molecules.
Lili Zhao, Nanjing Tech University, China, Gernot Frenking, Nanjing Tech University and University of Marburg, Germany, Mingfei Zhao, Fudan University, Shanghai, China, and colleagues have examined the validity of these heuristic rules for the structure and stability of molecules. The researchers studied the late lanthanide complexes [Ln(CO)8]– (Ln = Tm, Yb, Lu) using density functional theory (DFT) calculations. Simple counting of the electrons in these complexes gives 32 (Tm), 33 (Yb) and 34 (Lu) valence electrons, respectively.
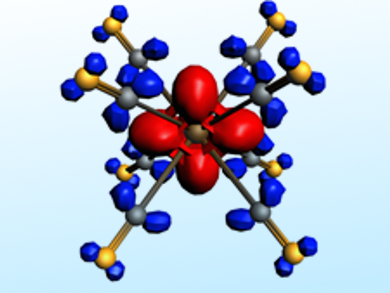
The nature of the metal–CO interactions has been analyzed using calculations with an energy decomposition method. This method provides qualitative and quantitative insights into the interatomic interactions. The picture above, for example, shows charge flow from the metal d orbital (red region) to the CO ligands (blue region) in [Lu(CO)8]−. It was possible to show that the 32-electron rule applies to all three systems if only those electrons that actually occupy the valence orbitals of the metal are considered.
- Octacarbonyl Anion Complexes of the Late Lanthanides Ln(CO)8– (Ln = Tm, Yb, Lu) and the 32-Electron Rule,
Jiaye Jin, Sudip Pan, Xiaoyang Jin, Shujun Lei, Lili Zhao, Gernot Frenking, Mingfei Zhou,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201805260