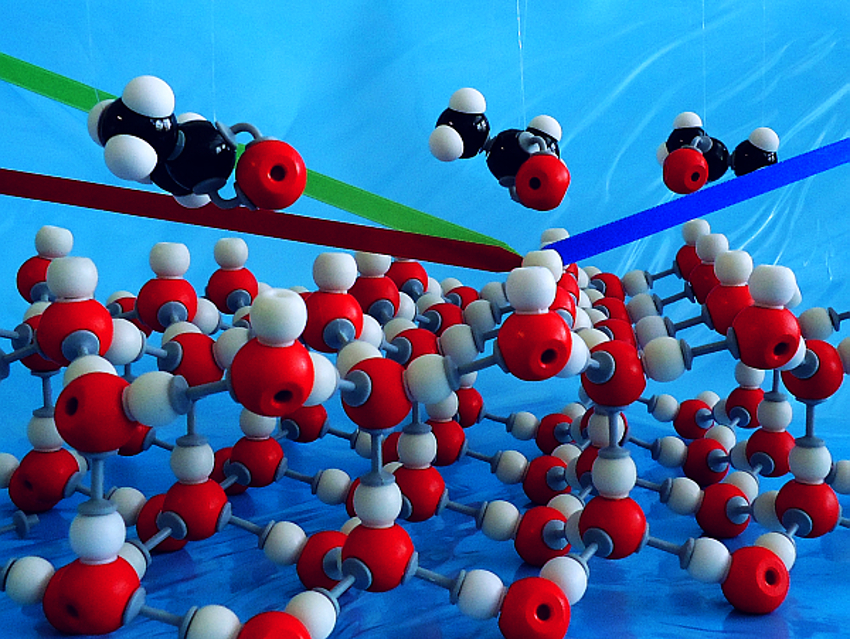
The interaction between small oxygenated organic molecules such as acetone and water or ice surfaces plays an important role in the formation of HOx radicals in the atmosphere. These HOx (i.e. OH and peroxy) radicals disturb the ozone–oxygen cycle and lead to the depletion of the ozone layer. However, little is known about the interactions of molecules such as acetone with ice on a molecular level.
Jenée D. Cyran, Marc‐Jan van Zadel, and Mischa Bonn, Max Planck Institute for Polymer Research, Mainz, Germany, Ellen H. G. Backus, Max Planck Institute for Polymer Research and University of Vienna, Austria, have studied the adsorption of acetone on water and ice surfaces. The team used surface-specific vibrational sum frequency generation (SFG) spectroscopy to gain molecular-level insights into the adsorption process. For the study of acetone interacting with a water surface, the researchers used mixtures of acetone in water contained in a Teflon container. For studying the interaction of acetone with ice, they used a flow cell, which enabled a controlled flow of acetone vapor over the ice surface.
Using the vibrations of the acetone and interfacial water molecules as indicators, the researchers found that acetone interacts very differently with the two surfaces. The interaction with ice primarily occurs between acetone and free O−H groups, increasing the strength of interfacial water–water hydrogen bonds. For liquid water, the interaction seems less specific and leads to an overall weakening of the interfacial water hydrogen bonds.
This result is in agreement with the different photoreactivities that had been determined previously. Understanding these interactions on the molecular level could help to provide better models for the rapidly changing atmospheric composition.
- Comparative Adsorption of Acetone on Water and Ice Surfaces,
Jenée D. Cyran, Ellen H. G. Backus, Marc-Jan van Zadel, Mischa Bonn,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201813517