Dynamic kinetic resolution (DKR) is an approach to transform racemic reagents into products enriched with one stereoisomer. Olefin hydroacylation is a direct method to construct ketones from aldehydes and olefins. The kinetic resolution of aldehydes using hydroacylation is known; however, the scope and theoretical yield of such transformations are limited.
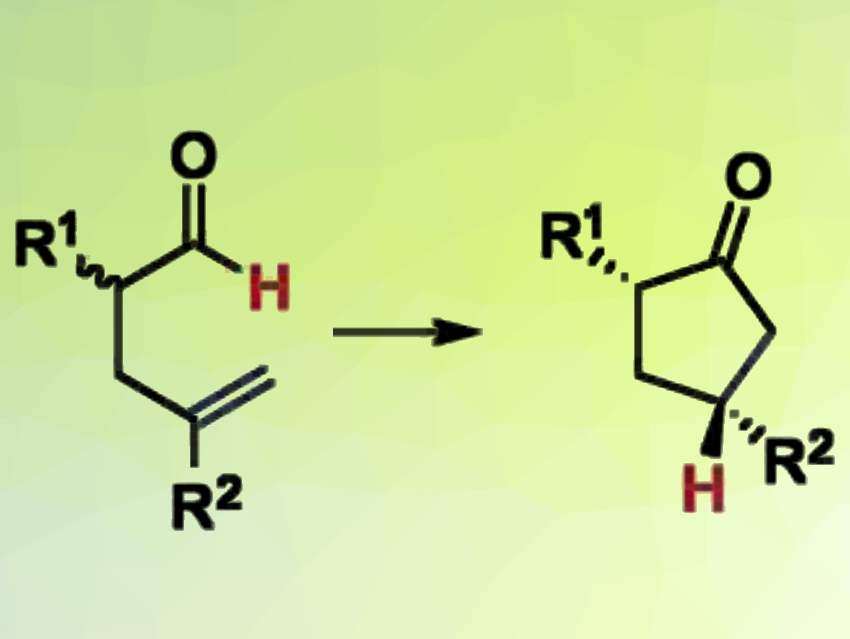
Vy M. Dong, University of California, Irvine, USA, and colleagues have developed a DKR of racemic α-allyl aldehydes, coupled with hydroacylation, to synthesize chiral α,γ-disubstituted cyclopentanones with high enantio- and diastereoselectivities. The team used two catalysts to transform a mixture of aldehyde enantiomers into enantioenriched cyclic ketones (reaction pictured).
An achiral amine catalyst continually interconverts the two enantiomers of the aldehyde. A chiral cationic rhodium catalyst removes the formed (R)-enantiomer from the equilibrium by transforming it into the desired optically pure cyclopentanone, which does not react any further.
The scope of the method extends to three different classes of α-allyl aldehyde reagents. The DKR method produces useful building blocks for drug discovery and natural product synthesis: chiral cyclopentanones and cyclopentanes are common in biologically relevant molecules.
- Dynamic Kinetic Resolution of Aldehydes by Hydroacylation,
Zhiwei Chen, Yusuke Aota, Hillary M. H. Nguyen, Vy M. Dong,
Angew. Chem. Int. Ed. 2019, 58, 4705–4709.
https://doi.org/10.1002/anie.201900545


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
