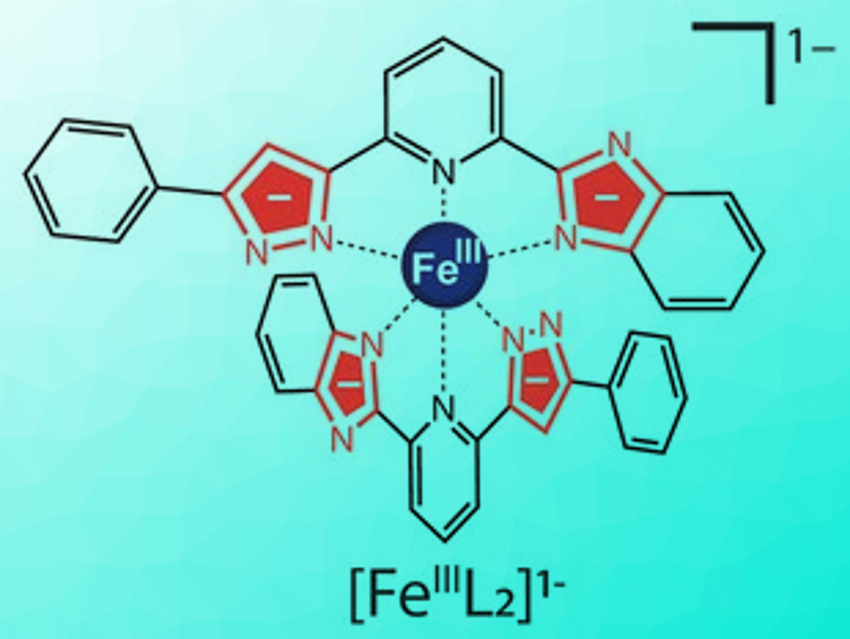
Molecules that can switch their electronic state in response to external stimuli are useful for next-generation molecular devices. Spin-crossover complexes are especially interesting in this regard. Careful tuning of the ligand field strength in such systems allows switching between two distinct magnetic states.
Takuya Shiga, Hiroki Oshio, University of Tsukuba, Japan, Graham N. Newton, University of Nottingham, UK, and colleagues have shown that a simple iron complex (pictured in its deprotonated form) can have unique multi-state switching properties thanks to a new type of unsymmetric “Brønsted ligand”. The ligand, H2L or 2‐[5‐phenyl‐1H‐pyrazole‐3‐yl]6‐benzimidazole pyridine, has two non-equivalent acidic protons, which can be removed one at a time. The stepwise addition or removal of protons to or from the ligands allows the in-situ modification of the ligand field strength in the complex. The team synthesized both Fe(II) and Fe(III) complexes using this ligand.
Five discrete physical states—each with unique electronic, magnetic, and optical properties—were generated from a single mononuclear iron complex. The states are interconvertible in both solution and solid state. in the researchers’ opinion, this strategy could inspire the development of new molecular switches, sensors, and optoelectronic devices.
- A Brønsted-Ligand-Based Iron Complex as a Molecular Switch with Five Accessible States,
Takuya Shiga, Ryo Saiki, Lisa Akiyama, Reiji Kumai, Dominik Natke, Franz Renz, Jamie M. Cameron, Graham N. Newton, Hiroki Oshio,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201900909