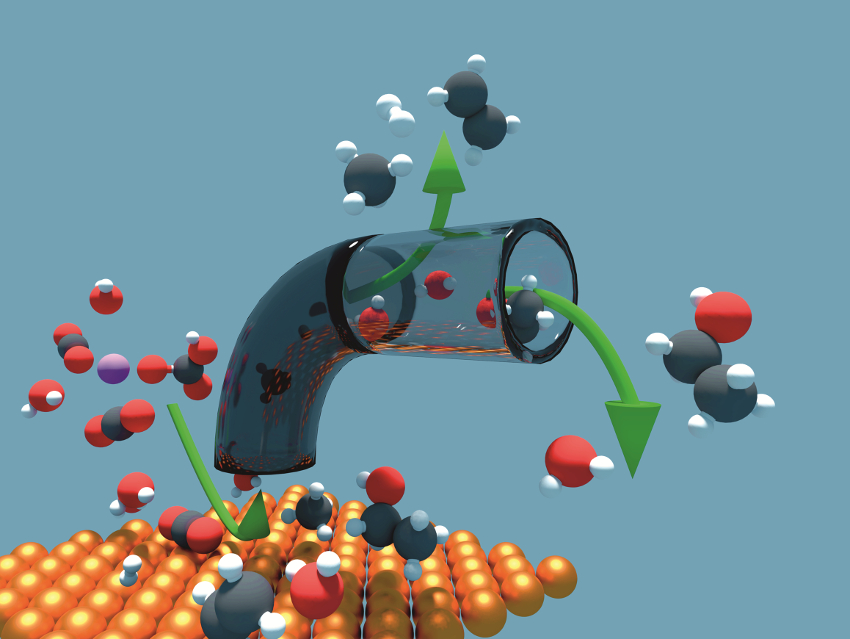
The electrochemical conversion of substrates to, e.g., fuels or added-value chemicals requires active and selective electrocatalysts. In some cases, the catalysts must also be robust to withstand dynamic reaction conditions. Real-time information about the behavior of catalytic systems under such dynamic conditions is important for the optimization of catalysts.
Peyman Khanipour, Forschungszentrum Jülich and Friedrich-Alexander University Erlangen-Nürnberg, both Erlangen, Germany, Ioannis Katsounaros, Forschungszentrum Jülich, and colleagues have developed a method to continuously monitor reaction products during electrochemical reactions. The team combined two mass spectrometry (MS) techniques for real-time analysis of a product solution after passing through an electrochemical flow reactor.
During the reaction, some of the electrolyte—together with the products—is continuously withdrawn from the reactor using a capillary. Gaseous products are then extracted using molecular diffusion through a hydrophobic membrane. The gases are analyzed using an electron ionization quadrupole mass spectrometer (EI‐QMS). The remaining liquids are nebulized using a high‐flow gas stream and the resulting fine mist is ionized with a direct analysis in real time (DART) ion source. Time-of-flight mass spectrometry (TOF-MS) is then used for the detection of the ionized species.
Compared to classical methods, this approach offers an improved time resolution for the detection of formed liquids and gases. This could allow rapid investigations of materials and conditions for electrochemical synthesis because of the significantly decreased analysis times. In addition, the transient formation of products due to dynamic reaction conditions can be monitored. The method could be useful for the development of robust processes for selective electrochemical conversions.
- Electrochemical real-time mass spectrometry (EC-RTMS) – monitoring electrochemical reaction products in real time,
Peyman Khanipour, Mario Löffler, Andreas M. Reichert, Felix T. Haase, Karl J. J. Mayrhofer, Ioannis Katsounaros,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201901923