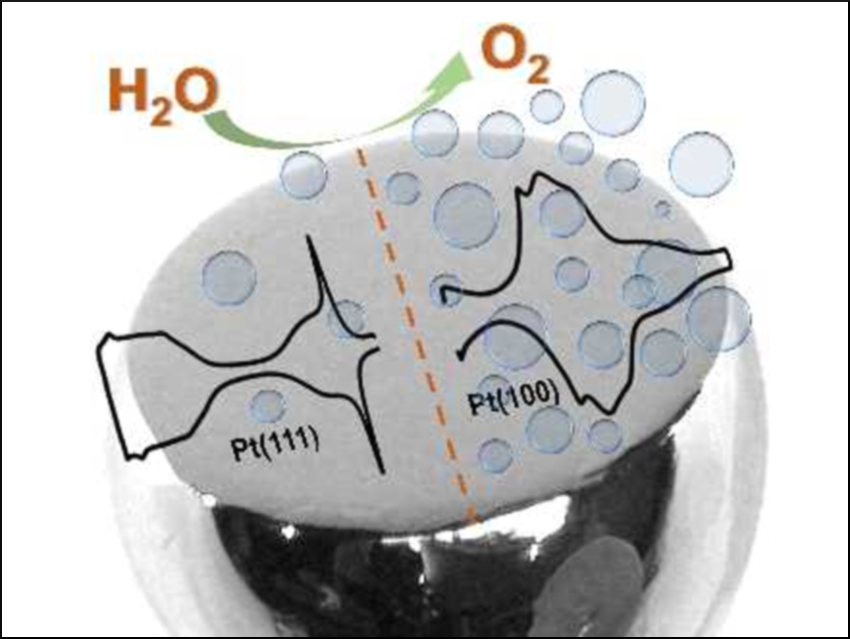
The oxygen evolution reaction (OER) is the anode process in many electrolysis cells. It generally suffers from slow kinetics that limit the efficiency.
Matthias Arenz, University of Bern, Switzerland, and colleagues have studied the OER on Pt(111) and Pt(100) single-crystal model electrodes to understand fundamental structure-activity relationships. The team performed electrochemical experiments under conditions that partially maintain an ordered surface structure. They showed that Pt(100)—despite its lower surface atomic density—produces about ten times more oxygen than Pt(111).
Density functional theory (DFT) calculations suggest that this difference is caused by the formation of 3D platinum oxide phases. With its open structure, Pt(100) cannot retain metastable oxygen in the subsurface. The more dense Pt(111) surface allows the formation of a Pt–O layer with reduced conductivity compared to metallic Pt. These results imply that the OER performance can be enhanced by engineering surface structures that prevent the formation of 3D oxide layers.
- Examining the structure sensitivity of the Oxygen Evolution Reaction on Pt single crystal electrodes: a combined experimental and theoretical study,
Francesco Bizzotto, Hassan Ouhbi, Yongchun Fu, Gustav Wiberg, Ulrich Aschauer, Matthias Arenz,
ChemPhysChem 2019.
https://doi.org/10.1002/cphc.201900193