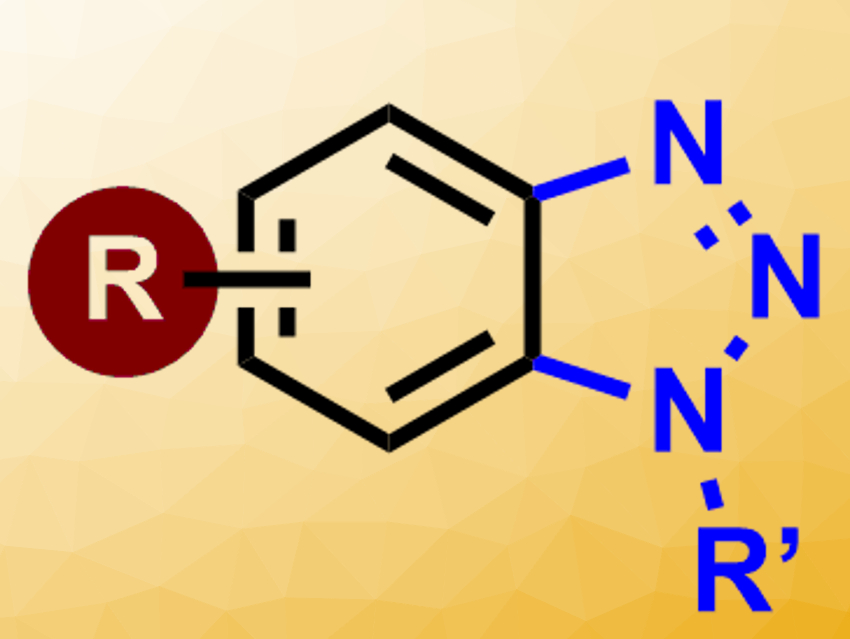
Benzotriazoles (pictured above) are widely used in organic synthesis and materials sciences. They also are core components of a large number of pharmaceutically active agents, including antifungal, anticancer, and antiparasitic drugs. Traditional methods for the synthesis of this heterocycle involve harsh acidic conditions, and some approaches lack regioselectivity for the preparation of N-substituted derivatives.
Andrew Sutherland and colleagues, University of Glasgow, UK, have developed a particularly mild method for the general synthesis of benzotriazoles from readily available 1,2-aryldiamines. The reaction (pictured below) uses a polymer-supported nitrite reagent and p-tosylic acid. It proceeds via a diazotization, followed by cyclization.
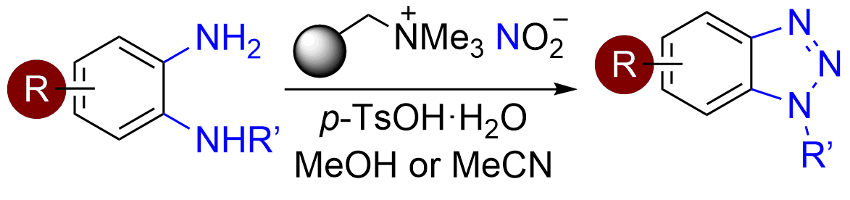
The method works under mild conditions. It is used for the preparation of a wide range of N-alkyl-, aryl- and acyl-substituted benzotriazoles. It allows the synthesis of pharmaceutically important compounds. The team also demonstrated the potential of this procedure for applications in bioorganic chemistry with the synthesis of a new, benzotriazole-containing α-amino acid.
- Synthesis of Structurally Diverse Benzotriazoles via Rapid Diazotization and Intramolecular Cyclization of 1,2-Aryldiamines,
Reka Faggyas, Nikki Sloan, Ned Buijs, Andrew Sutherland,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900463

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

