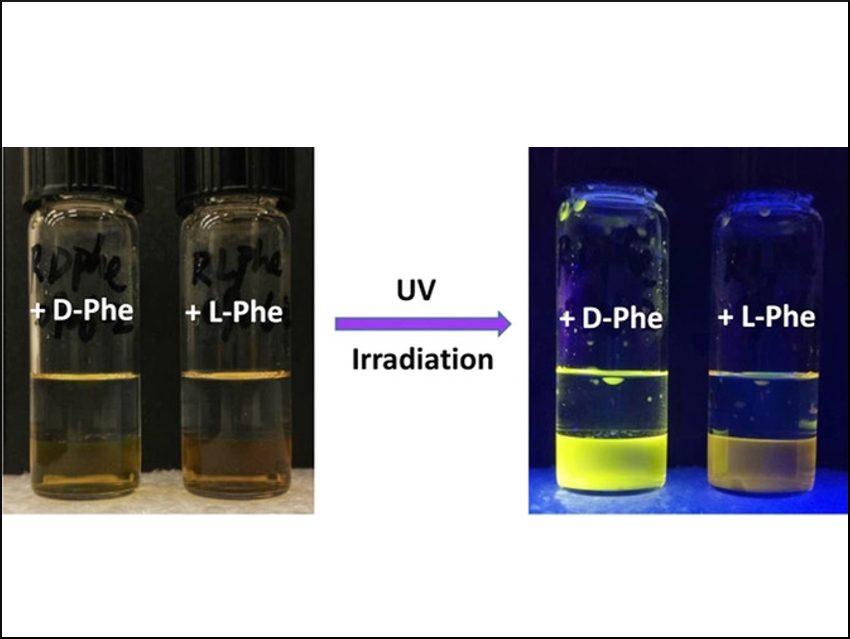
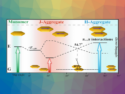
The enantioselective fluorescence recognition of chiral organic compounds could have applications, for example, in high-throughput screenings of asymmetric reactions. One challenge for this method, however, is the possible interference of various components of a reaction mixture on the fluorescence measurement.
Shuang-Xi Gu, University of Virginia, Charlottesville, USA, and Wuhan Institute of Technology, China, Lin Pu, University of Virginia, and colleagues have developed fluorescent probes for the enantioselective recognition of chiral molecules. The team used the hydrophobic and fluorophilic properties of highly fluorinated probes to minimize their interference with the components of a reaction mixture. The probes are perfluoroalkyl-substituted bis(binaphthyl) compounds. They were prepared from a 2,6‐dibromomethyl pyridine with a highly fluorinated alkyl chain and two equivalents of an enantiopure 1,1’‐binaphthyl‐based aldehyde.
In the presence of Zn(II) and tetrabutylammonium hydroxide (TBAH), the probe shows highly enantioselective fluorescence responses (example pictured) toward structurally diverse amino acids. This effect was observed in the organic phase of a biphasic system composed of water and the flourous solvent 1H,1H,2H,2H‐perfluoro‐1‐octanol (PFOH). The probe provides enantiomeric fluorescence enhancement ratios up to 45.2 (for histidine).
The researchers attribute the enantioselectivity to the formation of imine–Zn(II) complexes via a condensation of the aldehyde groups of the probe with the amino acids. These complexes have different stabilities for the different combinations of binaphthyl- and amino acid enantiomers. According to the team, this is the first example of fluorescent separation of the enantiomers of amino acids using a fluorous-phase-based separation process.
- Biphasic Enantioselective Fluorescent Recognition of Amino Acids by a Fluorophilic Probe,
Yuan-Yuan Zhu, Xue-Dan Wu, Mehdi Abed, Shuang-Xi Gu, Lin Pu,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201900880