Quinazolinones are found in many pharmaceutically relevant natural products. They have versatile biological activities. However, methods for the synthesis of fused polycyclic quinazolinone derivatives are rare, especially metal‐free approaches.
Ke Zheng, Sichuan University, Chengdu, China, and colleagues have developed an efficient, metal-free, (NH4)2S2O8-mediated intramolecular oxidative cyclization for the construction of fused polycyclic quinazolinone derivatives under mild conditions. The team started from 2‐aminobenzamides and used (NH4)2S2O8 as an oxidant to give the desired quinazolinones in good to excellent yields.
During the reaction, the sp3 C–H bond at the α-position of the amide is oxidized by an SO4·– radical anion, which is generated in situ from (NH4)2S2O8 under thermolysis. The formation of a C–N bond with the amino group then generates the product.
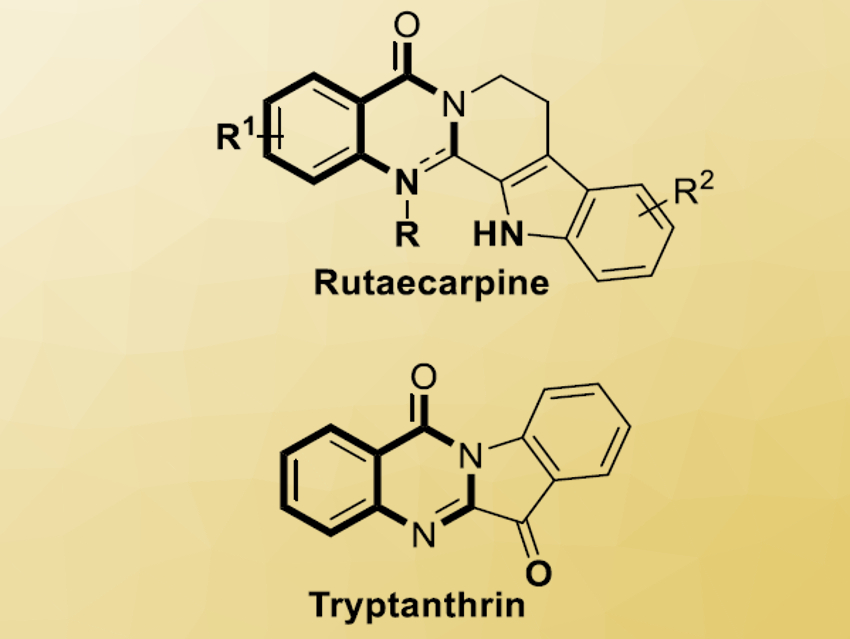
The reaction led to a series of polycyclic quinazolinone derivatives with good functional group tolerance. The natural products tryptanthrin and rutaecarpine (pictured), as well as their derivatives, were easily synthesized by this strategy.
- Metal-Free Synthesis of Polycyclic Quinazolinones Enabled by a (NH4)2S2O8-Promoted Intramolecular Oxidative Cyclization,
Lijuan Xie, Cong Lu, Dong Jing, Xinrui Ou, Ke Zheng,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900469