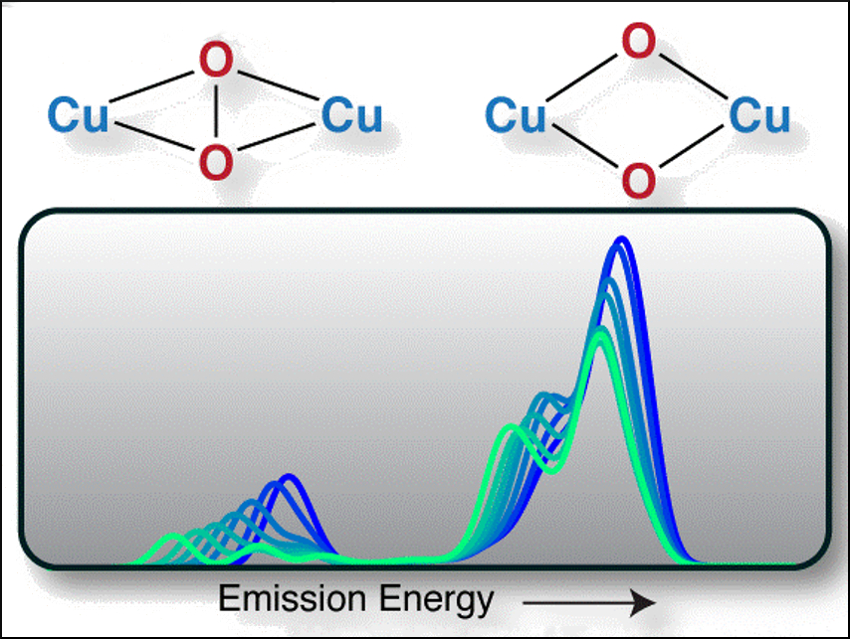
The process of breaking or forming O–O bonds is important for respiration, photosynthesis, and many catalytic reactions in the context of energy conversion. Complexes of transition metals such as copper can activate O2. Dicopper centers can, for example, cleave the O–O bond of a peroxo ligand with a low activation barrier (pictured).
Serena DeBeer, Max Planck Institute for Chemical Energy Conversion, Mülheim an der Ruhr, Germany, William B. Tolman, University of Minnesota, Minneapolis, USA, and colleagues have studied two dicopper-oxygen complexes using Valence-to-Core X-ray emission spectroscopy (VtC XES). The complexes represent two extremes of O–O bond activation where the bond is either retained or cleaved. The peroxo complex with an O−O bond (core pictured left) was supported by N,N‘‐di‐tert‐butyl‐ethylenediamine (DBED) ligands. The bis(μ‐oxo) complex with a cleaved bond (core pictured right) was supported by 1,4,7‐trimethyl‐1,4,7‐triazacyclononane (Me3TACN) ligands.
Due to the sensitivity of VtC XES to the environment of the copper ligand, the complexes have distinct spectra resulting from the differing oxygen cores. The technique can, thus, directly detect the presence of an O–O bond in a copper-peroxo complex. According to the researchers, these results could be the foundation for future time‐resolved studies of molecular copper oxygen chemistry in both model complexes and enzymes.
- Valence-to-Core X-ray Emission Spectroscopy as a Probe of O–O Bond Activation in Cu2O2 Complexes,
George E. Cutsail, Nicole L. Gagnon, Andrew D. Spaeth, William B. Tolman, Serena DeBeer,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201903749