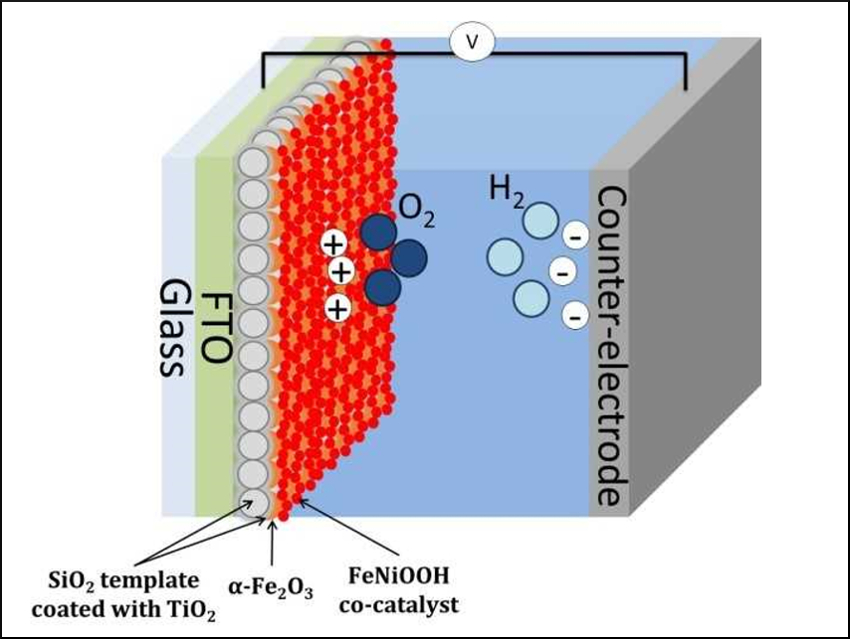
Hematite (α-Fe2O3) is a promising semiconductor for photoelectrochemical (PEC) cells, mainly due to its abundance, low cost, and excellent long-term stability. However, the performance of α-Fe2O3 photoelectrodes is limited by the low hole mobility and the fast electron-hole recombination. Nanostructuring and host-guest structures could help to solve these problems.
Paula Dias and colleagues, Universidade do Porto, Portugal, have prepared a hematite host-guest photoelectrode for solar water-splitting. The electrode is based on a nanostructured SiO2-TiO2 host scaffold (pictured in grey). To prepare the scaffold, an SiO2 template was combined with a TiO2 thin conductive layer using atomic layer deposition. This support structure was coated with a layer of α-Fe2O3 (pictured in orange) using spray pyrolysis. The hematite film was coated with FeNiOOH as a co-catalyst (pictured in red).
Using this host-guest electrode, a photocurrent increase of ca. 85 % was observed compared with a standard α-Fe2O3 film. The SiO2 base provides a large surface area and the TiO2 thin layer acts as an efficient conductive charge collector The photoelectrode shows improved charge transfer kinetics and minimized electron-hole recombination in α-Fe2O3. This results in an improved water oxidation efficiency. According to the researchers, the SiO2-TiO2 scaffold could be a promising support structure for other charge-transport-limited materials.
- Synthesis of Host-Guest Hematite Photoelectrodes for Solar Water Splitting,
Filipe Francisco, Paula Dias, Dzmitry Ivanou, Fátima Santos, João Azevedo, Adélio Mendes,
ChemNanoMat 2019.
https://doi.org/10.1002/cnma.201900141