Triarylboranes are excellent π-acceptors (A) due to the empty p-orbital on boron. They can be used to prepare efficient A-π-A compounds for nonlinear optics. Such quadrupolar dyes have highly desirable two-photon absorption properties. However, they need to be water-soluble and water-stable to be efficient chromophores for fluorescence imaging of live cells. The challenge is to protect the unsaturated boron center from attack by water or other nucleophiles, while maintaining or enhancing the optical properties.
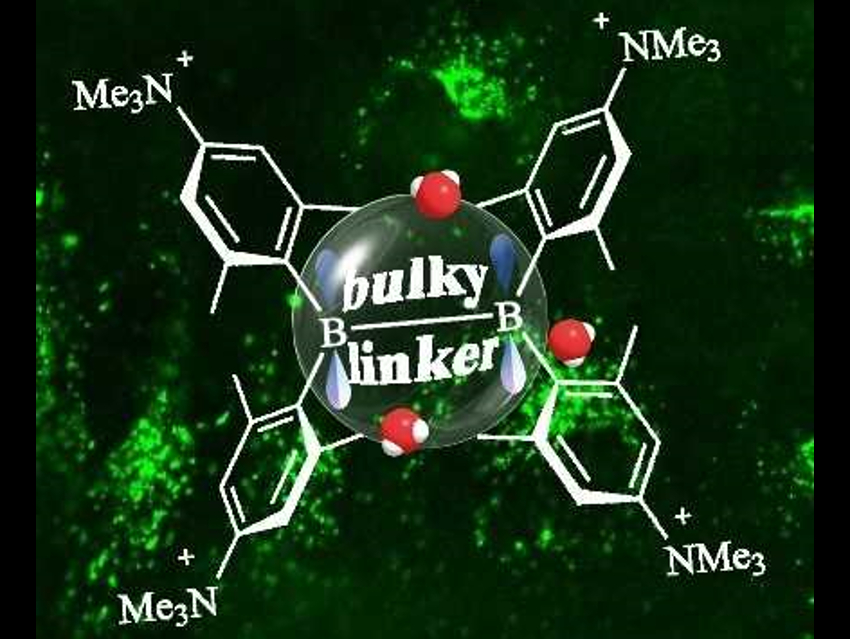
Shigehiro Yamaguchi, Nagoya University, Japan, Todd B. Marder, University of Würzburg, Germany, and colleagues have studied triarylboranes for live-cell imaging. They attached ammonio substituents at the acceptor group to provide water solubility. The resulting triarylaboranes have a (para‐(N,N,N‐trimethylammonio)xylyl)2B–(linker)–B(para‐(N,N,N‐trimethylammonio)xylyl)2 structure (pictured). The team studied the steric requirements for the π-linker in these A-π-A dyes by varying the linker and using X-ray diffraction, variable-temperature-NMR measurements, and UV/Vis absorption spectroscopy in water.
Using bulky π-linkers such as anthracene or xylylene, the researchers prepared dyes which are water-stable for at least 48 h, while maintaining a very high fluorescence quantum yield. These highly water-stable and water-soluble dyes can be used for two-photon excited fluorescence imaging of live cells.
- Optimization of Aqueous Stability versus π-Conjugation in Tetracationic Bis(triarylborane) Chromophores: Applications in Live-Cell Fluorescence Imaging,
Stefanie Griesbeck, Matthias Ferger, Corinna Czernetzi, Chenguang Wang, Rüdiger Bertermann, Alexandra Friedrich, Martin Haehnel, Daniel Sieh, Masayasu Taki, Shigehiro Yamaguchi, Todd B. Marder,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201900723