Various members of the statin family (e.g., atorvastatin or fluvastatin) can be used as drugs to treat abnormal lipid levels and prevent cardiovascular disease. Common features of their structures are functionalized side chains that are derived from (3R,5R)-3,5-dihydroxyheptanoic acid. Because of their resemblance to mevalonic acid, statins are suitable inhibitors of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. This enables them to modulate the levels of cholesterol and other lipids. Statins can also possess anti-inflammatory and immunomodulating properties.
Christine Beemelmanns, Leibniz Institute for Natural Product Research and Infection Biology – Hans-Knöll-Institute (HKI), Jena, Germany, and Leipzig University, Germany, and colleagues have developed a synthetic procedure to generate functionalized δ-hydroxy-β-keto-esters with high enantioselectivity and in good yields. The approach involves an asymmetric Mukaiyama aldol reaction protocol. The team used a chiral Lewis acid complex with BINOL ligands for the reaction of protected enolates with aldehydes in the presence of LiCl. The desired products were obtained in moderate to good yields and with excellent enantioselectivities.
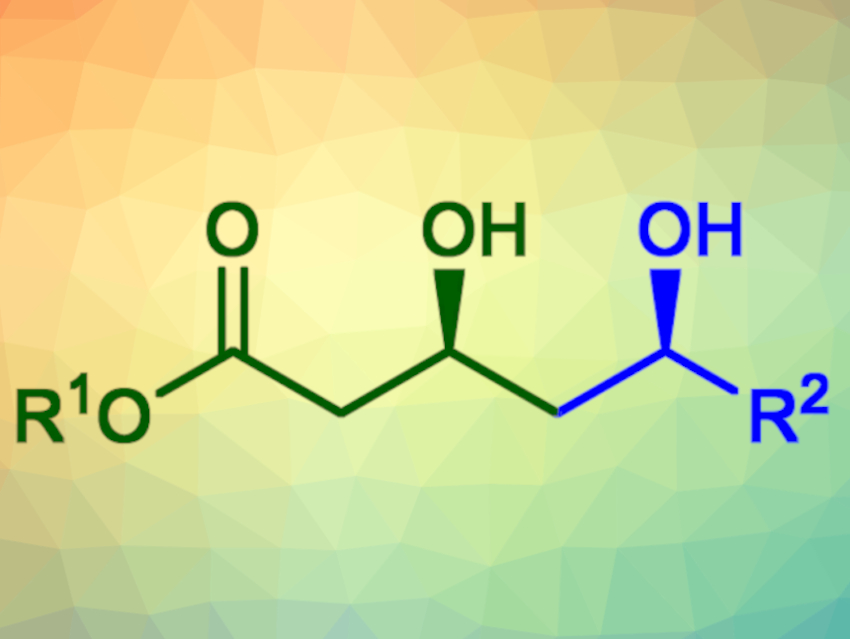
The obtained δ-hydroxy-β-keto-esters were reduced diastereoselectively using sodium borohydride in the presence of a chelating agent to give the corresponding syn-diols (example pictured) in yields of 77–96 %. The corresponding anti-diols could be generated in yields of 50–97 % using Me4NHB(OAc)3 as a reducing agent instead.
Some members of the statin family are known as modulators of the 5-lipoxygenase (5-LOX) enzyme, which generates leukotrienes (LTs) from arachidonic acid. Therefore, the team tested the synthesized compounds for their 5-LOX modulating activity and found a reduced production of proinflammatory LTs. The mechanism of this inhibitory process is unknown so far but will be a subject of future studies.
- Synthesis of functionalized δ‐hydroxy‐β‐keto esters and evaluation of their anti‐inflammatory properties,
Michel Grosse, Kerstin Günther, Paul M. Jordan, Dávid Roman, Oliver Werz, Christine Beemelmanns,
ChemBioChem 2022.
https://doi.org/10.1002/cbic.202200073