The regioselective bromination of phenols can be challenging. This is due to the very small electronic differences between the para-position and the ortho-position of phenols. Mixtures of HBr and dimethyl sulfoxide (DMSO) or TMSBr and DMSO (TMS = trimethylsilyl) are sometimes used to brominate phenols. However, they give the toxic dimethylsulfide as a byproduct and the selectivity could be improved.
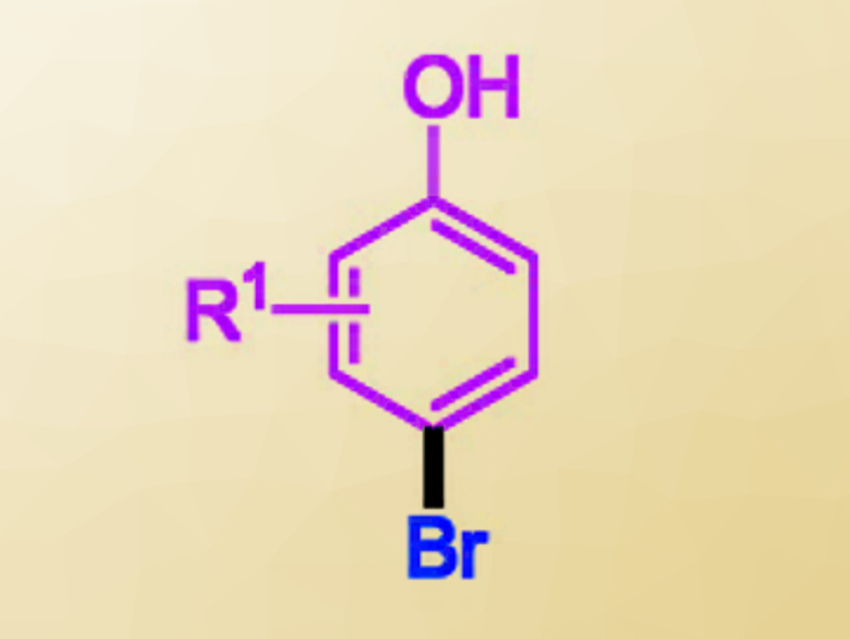
Xiantao Ma, Lin Tang, Qiuju Zhou, Xinyang Normal University, China, and colleagues have developed a mild and regioselective bromination of phenols with TMSBr. The team replaced the commonly used DMSO with sulfoxides that have bulkier substituents. The resulting thioether byproducts promote the reaction. The best results were obtained when using (4‐ClC6H4)2SO as the sulfoxide and acetonitrile as the solvent at room temperature. The desired para-brominated phenols (pictured) were obtained from the corresponding phenols and TMSBr in high selectivities (up to 99/1).
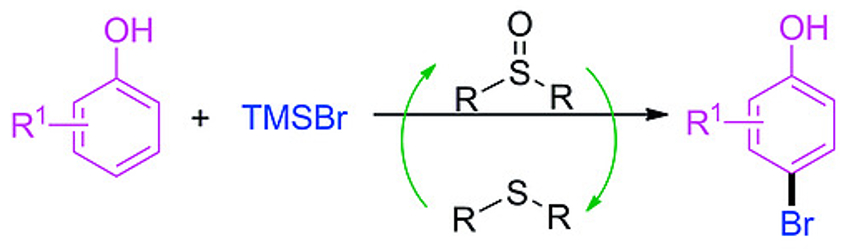
This protocol is easy to scale up. The thioether byproduct can be recycled and the desired product can be isolated without column chromatography. According to the researchers, the high regioselectivity of the reaction could be caused by the interaction between the thioether and phenol via an O–H···S hydrogen bond. This causes a preference for the bromination of the para-position.
- Mild and Regioselective Bromination of Phenols with TMSBr,
Xiantao Ma, Jing Yu, Mengyuan Jiang, Mengyu Wang, Lin Tang, Mengmeng Wei, Qiuju Zhou,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900794
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)


