Imine hydrosilylation is a valuable tool for the catalytic conversion of imines to amines. It is usually catalyzed by transition-metal complexes. However, replacing transition metals with more abundant s-block metals would improve the reaction’s sustainability. While alkene and ketone hydrosilylations catalyzed by group 2 metals are well-studied, little had been known about imine hydrosilylation with these catalysts so far.
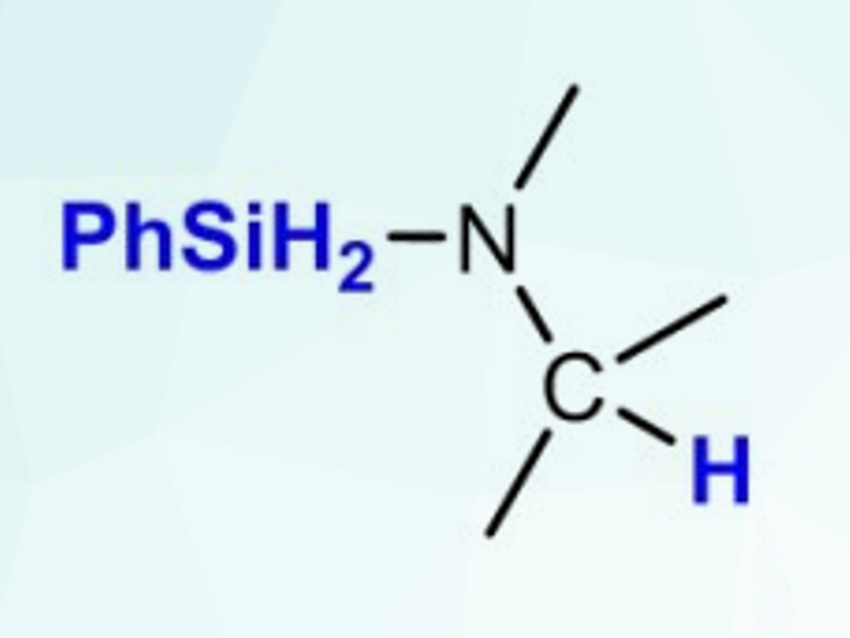
Sjoerd Harder and colleagues, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, have achieved an efficient catalytic reduction of imines with phenylsilane (PhSiH3, product pictured) using the potassium-, calcium-, and strontium-based catalysts [(DMAT)K(THF)]∞, (DMAT)2Ca⋅(THF)2, and (DMAT)2Sr⋅(THF)2 (DMAT = 2‐dimethylamino‐α‐trimethylsilylbenzyl). Different aldimines and the more challenging ketimine substrate Ph2C=NPh were reduced at temperatures between 25–60 °C with catalyst loadings down to 2.5 mol%.
Simple amides like KN(SiMe3)2 or M[N(SiMe3)2]2 (M = Ca, Sr, Ba) can also catalyze this reaction. The team found that the reactivity increases along the row K < Ca < Sr < Ba. The mechanism of this reaction could be either a metal-hydride mechanism (as in alkene hydrosilylation) or a silicate mechanism (as in ketone hydrosilylation). The team showed that imine hydrosilylation follows the metal-hydride mechanism using reactions with a catalyst model system, the isolation of intermediates, and density functional theory (DFT) calculations.
This work shows that s-block metals can efficiently catalyze the hydrosilylation of imines via a metal-hydride mechanism. This offers an alternative reaction path for substrates where direct hydrogenation is not feasible, while avoiding toxic or expensive transition metals as catalysts.
- Early Main Group Metal Catalysts for Imine Hydrosilylation,
Holger Elsen, Christian Fischer, Christian Knüpfer, Ana Escalona, Sjoerd Harder,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201904148