Metathesis is a type of reaction that allows alkenes or alkynes to “swap” substituents. It can be used, e.g., in the synthesis of unsaturated organic compounds or for polymerizations. Generally, transition-metal complexes based on W, Mo, Ru, etc. are used to catalyze olefin metathesis. Ruthenium-based complexes are particularly common in homogenous catalysis. To be usable in environmentally friendly protic solvents, these catalysts need to be modified—for example, by introducing charged functional groups. However, existing systems that use this approach require high catalyst loadings, which makes them impractical for industrial use.
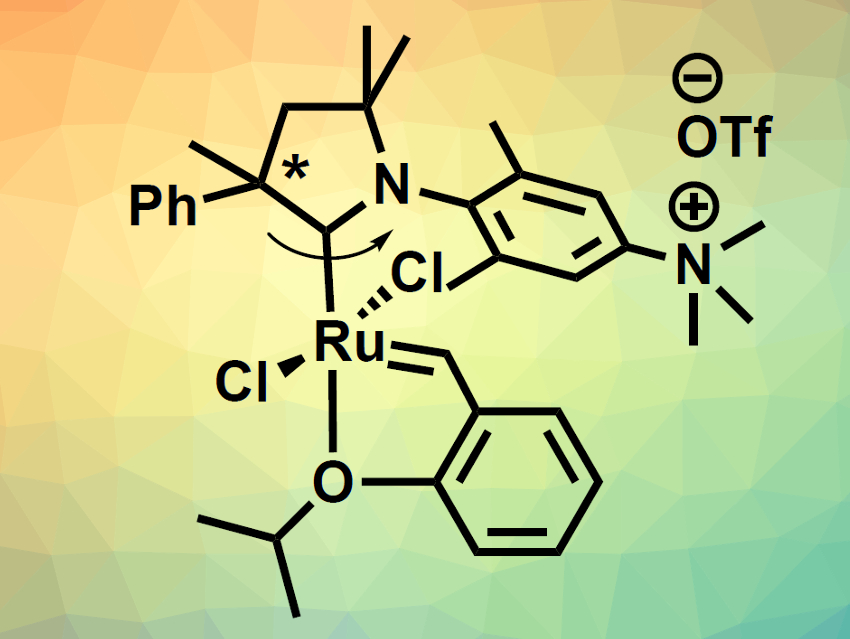
Róbert Tuba, Research Centre for Natural Sciences of the Hungarian Academy of Sciences, Budapest, and colleagues have developed two ruthenium-based olefin metathesis catalysts containing cationic cyclic alkyl amino carbene (CAAC) ligands (pictured). The team first prepared the CAAC precursor in five steps from N,N-dimethyl-3,5-dimethylaniline, 2-phenylpropionaldehyde, and 3-chloro-2-methyl-1-propene. This precursor was converted to the corresponding carbene in situ while it was reacted with existing Ru metathesis catalysts. This allowed the team to perform a ligand exchange. Finally, the ligand’s dimethylamino groups were methylated to give the desired charged trimethylammonium groups.
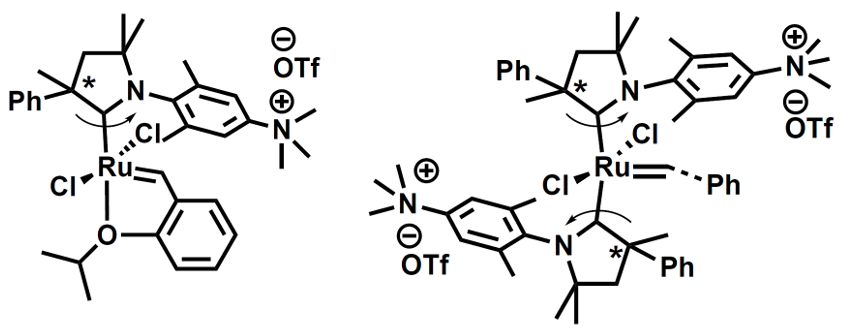
The team synthesized both a complex with a single CAAC ligand (pictured below on the left, 70 % yield) and a bis-carbene complex (pictured below on the right, 48 % yield). The complexes are soluble in protic solvents. They are stable and have high catalytic activities. The team found that the system can be used for metathesis reactions with catalyst loadings as low as 0.05 mol %, e.g., in methanol, isopropanol, water, or an ethanol/water mixture.
- Towards Sustainable Catalysis ‐ Highly Efficient Olefin Metathesis in Protic Media Using Phase Labelled Cyclic Alkyl Amino Carbene (CAAC) Ruthenium Catalysts,
Robert Tuba, Márton Nagyházi, Gábor Turczel, Áron Balla, Gábor Szálas, Imre Tóth, Gyula Tamás Gál, Petra Bombicz, Paul T. Anastas,
ChemCatChem 2020.
https://doi.org/10.1002/cctc.201902258