Photoswitches are molecules that reversibly isomerize when irradiated with light and change their shape and/or properties. Most organic photoswitches require UV light to trigger this isomerization, which can be destructive. However, photoswitches that respond to visible light often sacrifice other desirable properties, such as the life-time of the unstable isomer.
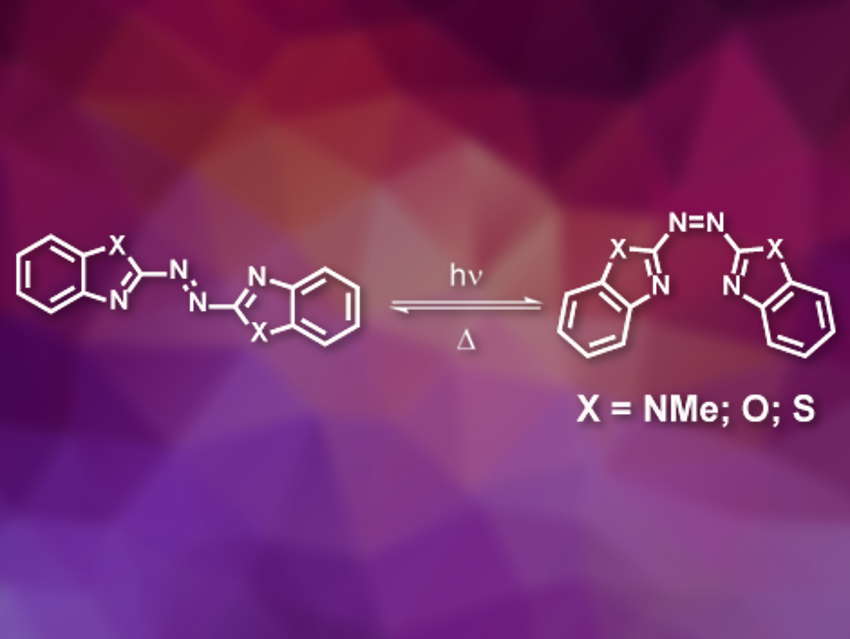
Jonathon E. Beves, University of New South Wales, Sydney, Australia, and colleagues have synthesized three new azobenzazole photoswitches (pictured), namely azobis(1‐methyl‐benzimidazole), azobis(benzoxazole), and azobis(benzothiazole). The compounds were prepared in one step by the oxidative coupling of two equivalents of the respective amino precursor using KMnO4 supported on FeSO4⋅7 H2O. The synthesis gave low to moderate yields of the desired photoswitches.
The compounds switch efficiently and repeatedly using visible light and return to their stable isomer over minutes at room temperature. The photoswitching of one of the azobenzazoles (X = NMe) can be modulated with acid: It absorbs yellow light when protonated and retains useful switching properties. The metastable isomer forms a strong intramolecular hydrogen bond. This provides an opportunity for sequestering protons and releasing them on demand, for example, to control catalysis using visible light.
- Visible‐Light Photoswitching by Azobenzazoles,
Aaron D. W. Kennedy, Isolde Sandler, Joakim Andréasson, Junming Ho, Jonathon E. Beves,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.201904309

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

