Therapeutically Interesting Toxin
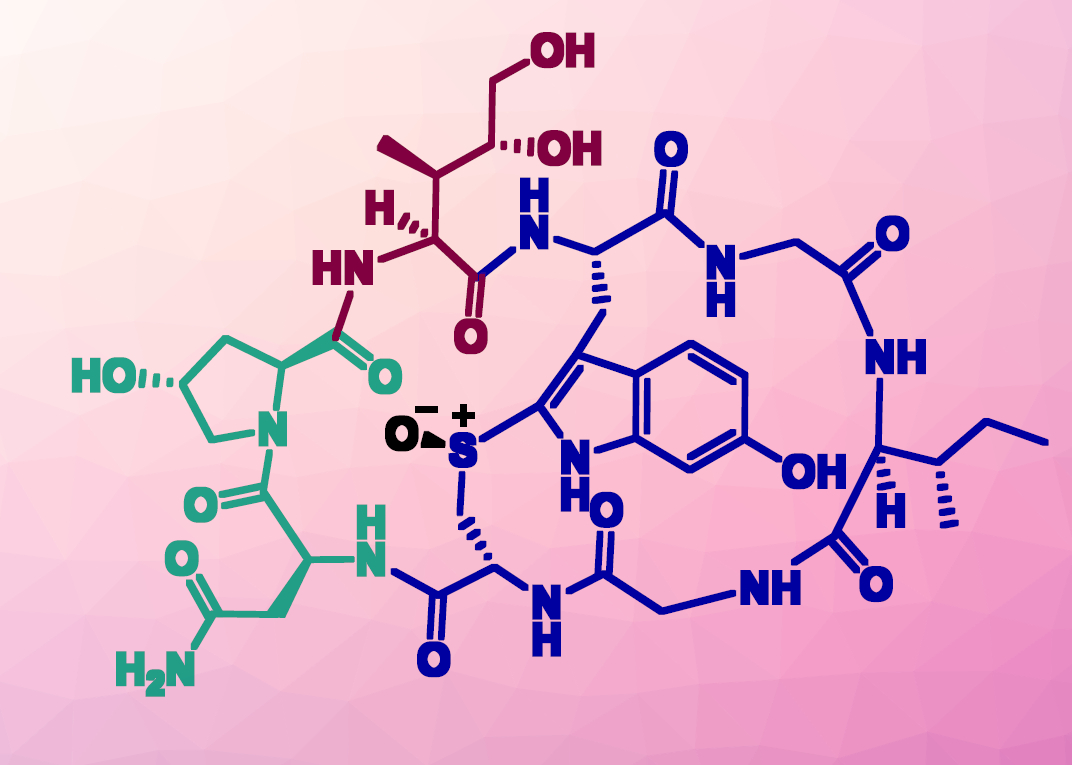
The death cap mushroom is highly toxic. However, some of its toxins can also be healing: amanitins are potential components for antibody-based cancer treatments. Roderich Süssmuth, Technical University Berlin, Germany, and colleagues have introduced a new synthetic route for α-amanitin. Their method seems suitable for production on a larger scale, finally making enough of the toxin available for further research.
Amanitins inhibit the enzyme RNA polymerase II with high selectivity, which leads to cell death. When transported into tumor cells by antibodies, the toxin could fight tumors. Until recently, however, the only source of amanitins was the mushrooms (Amanita phalloides) themselves, which limited the possibilities for experimentation.
New Synthetic Route for Amanitin
Some time ago, a total synthesis was reported for α-amanitin, the most powerful amanitin. The researchers have introduced an alternative route for a total synthesis that occurs entirely in the liquid phase, allows for the possibility of producing different structural variants, and can be implemented on a larger scale. “We decided to use a convergent route, meaning that several components are first synthesized independently and then finally put together to form the target molecule,” explains Süssmuth. The building blocks are three peptide fragments made of five, one, and two amino acids, respectively. The researchers refer to their method as a [5+1+2] synthesis.
Amatoxins are ring-shaped peptides made of eight amino acids that have an additional internal cross-ring bond between the amino acids tryptophan and cysteine, known as a tryptathionine. Instead of forming the required thioether bond at the end of their synthesis, the researchers made a building block from five amino acids that already contain the tryptathionine.
The key step for the formation of the other two peptide fragments was the development of routes for the production of the amino acids 6-hydroxytryptophan (Htp) and (3R,4R)-L-4,5-dihydroxyisoleucin (Dhil) in multigram quantities—a big challenge. Neither of these compounds is proteinogenic, meaning they are not coded in DNA. For this synthesis, they must be enantiomerically pure.
The researchers developed a seven-step synthesis for the production of Dhil—the shortest synthetic route to this type of amino acid reported to date. “We consider our new synthetic routes for Dhil and Htp to be industrially usable,” says Süssmuth. “Our α-amanitin synthesis is the first to be carried out entirely in the liquid phase. This offers access to larger amounts of α-amanitin for study as a potential cancer treatment. In addition, it could be the starting point for future industrial production of drugs based on amanitin.”
- A Convergent Total Synthesis of the Death Cap Toxin α-Amanitin,
Mary-Ann J. Siegert, Caroline H. Knittel, Roderich Süssmuth,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201914620