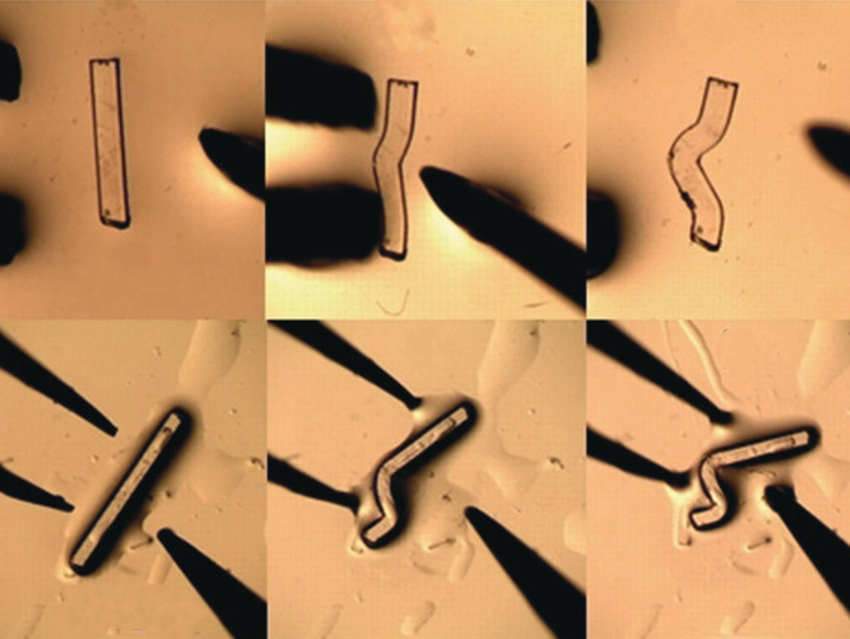
Flexible single crystals of covalently bound materials could be useful for technologies such as artificial muscles and mechanical actuators. However, the mechanics of mechanical flexibility in such materials is poorly understood.
Franziska Emmerling, Humboldt University of Berlin and BAM Federal Institute for Materials Research and Testing, both Berlin, Germany, and colleagues have discovered the first example of plastic flexibility in a 1D coordination polymer, [Zn(μ-Cl)2(3,5-dichloropyridine)2]n. The polymer was prepared from zinc(II)chloride and 3,5-dichloropyridine in ethanol. Crystals of the material were fully characterized in both their equilibrium and bent states using, e.g., atomic force microscopy (AFM), micro-focus X-ray diffraction, and terahertz time-domain (THz‐TD) spectroscopy.
Surprisingly, the flexibility of the polymer does not result from the bending or rupture of the covalent network. Instead, the stress of bending accumulates between the chains of the polymer. The researchers call this mechanism for mechanical flexibility “the spaghetti model” (see below). Mechanical stress first leads to slippage of polymers chains perpendicular to the bend. Other chains are then displaced parallel to the bend and become interwoven. The model does not yet include an understanding of the polymer’s structure at the ends of the macroscopically bent crystal.
.jpg)
- A Mechanistic Perspective on Plastically Flexible Coordination Polymers,
Biswajit Bhattacharya, Adam A. L. Michalchuk, Dorothee Silbernagl, Max Rautenberg, Thomas Schmid, Torvid Feiler, Klaus Reimann, Ahmed Ghalgaoui, Heinz Sturm, Beate Paulus, Franziska Emmerling,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201914798