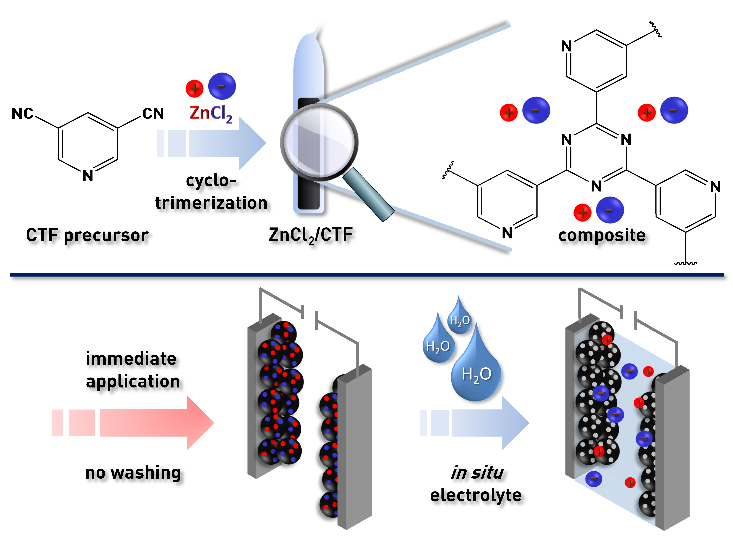
The synthesis of porous electrode materials often requires extensive purification steps, which results in large amounts of waste and low yields. Porous carbon materials are often used as abundant, cheap electrode materials. However, their surface properties are often not optimal for use with polar electrolytes, which leads to a need for modifications such as doping. Covalent triazine frameworks (CTFs) could be an alternative to porous carbon materials: They have adjustable properties that can be finely tuned by selecting a suitable monomer.
Lars Borchardt, Ruhr-Universität Bochum (RUB), Germany, Stefan Kaskel, Technische Universität Dresden, Germany, and colleagues have developed a CTF synthesis that avoids the need for purification. Instead, it uses the by-product as a valuable component for the final product—in this case, a supercapacitor. The CTFs were synthesized by a trimerization reaction of aromatic nitriles (pictured below) under ionothermal conditions in molten ZnCl2. In this process, ZnCl2 serves multiple purposes, namely, as the solvent, as the Lewis acid to trigger the trimerization, and as the porogen, i.e., the component that acts as a scaffold for the pores.
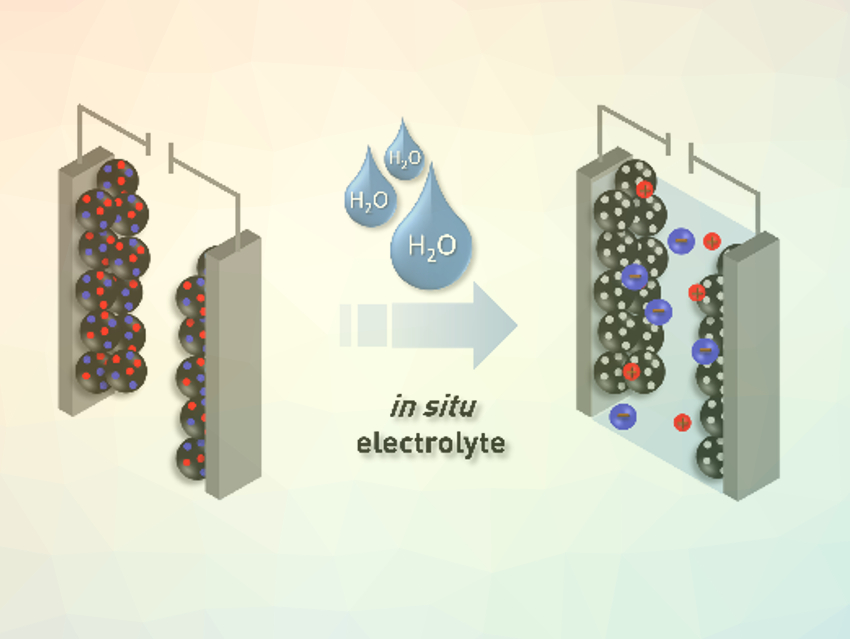
After the synthesis, the CTF material still contains a large amount of ZnCl2, which is dispersed finely throughout the whole sample. Removing this residue usually requires extensive washing procedures. However, the supercapacitor still needs an electrolyte in addition to the CTF electrode. The team used the ZnCl2 residue to create this electrolyte and avoid waste at the same time. The addition of water generates an aqueous ZnCl2 electrolyte in situ and simultaneously unblocks the pores of the CTF (pictured below). This approach circumvents extensive and time‐consuming washing steps and makes the process more environmentally friendly.
- In Situ Generation of Electrolyte inside Pyridine-based Covalent Triazine Frameworks for Direct Supercapacitor Integration,
Erik Troschke, Desirée Leistenschneider, Tilo Rensch, Sven Grätz, Johannes Maschita, Sebastian Ehrling, Benjamin Klemmed, Bettina V. Lotsch, Alexander Eychmüller, Lars Borchardt, Stefan Kaskel,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202000518