Acetals are important protecting groups for carbonyl compounds. The acetalization of carbonyl compounds is generally carried out via reactions with alcohols under acidic conditions. However, when the carbonyl compounds have another, acid-sensitive functional group, this acidic acetalization cannot be used. Therefore, methods for the non-acidic acetalization of carbonyl compounds can be useful.
Mitsiru Kitamura and colleagues, Kyushu Institute of Technology, Japan, have developed a new acetalization method for ketones and aldehydes under non-acidic conditions, using diazophenanthrenequinone as a reagent and PdBr2 as a catalyst. The reaction proceeds via the formation of a metal carbene, which is then attacked by the carbonyl compound.
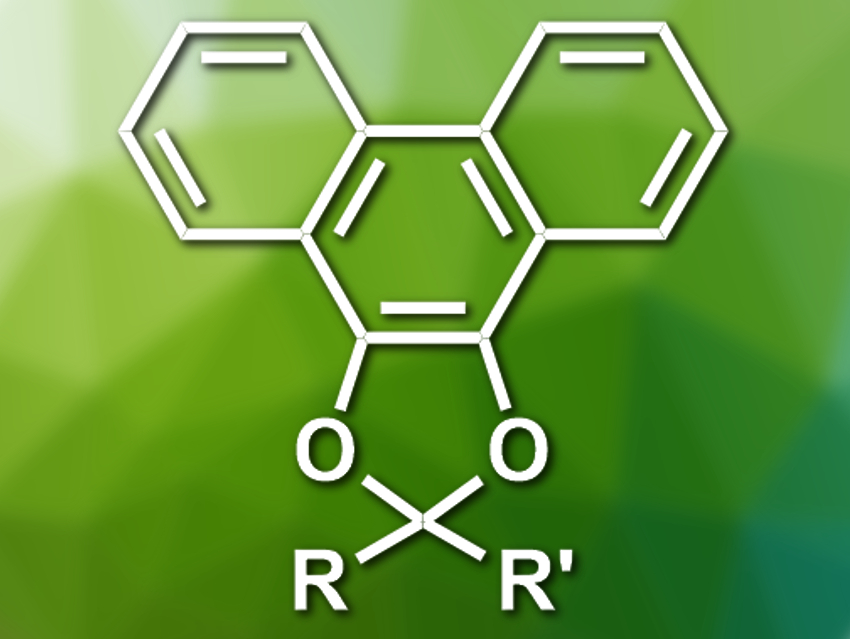
The resulting 9,10-phenanthrenedioxy acetals (pictured) are stable under mildly acidic conditions. The acetal groups can be removed under oxidative conditions using aqueous ceric ammonium nitrate (CAN) or under strongly acidic conditions to give the corresponding unprotected ketones and aldehydes.
The reactivity of the 9,10-phenanthrenedioxy acetals can be used for selective deprotections. For example, tert-butyldimethylsilyloxy protecting groups and 9,10-phenanthrenedioxy acetals can be selectively removed using either tetrabutylammonium fluoride (TBAF) or CAN, respectively. The 9,10-phenanthrenedioxy acetal protecting group can be used for a wide range of aldehydes and ketones.
- PdBr2-catalyzed acetal formation of carbonyl compounds using diazophenanthrenequinone: utility of 9,10-phenanthrenedioxy acetal,
Mitsuru Kitamura, Ryo Fujimura, Tomoaki Nishimura, Shuhei Takahashi, Hirokazu Shimooka, Tatsuo Okauchi,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000315


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
