Solid electrolytes are promising for the development of Li-metal batteries with high energy density and good safety. Among the solid electrolytes, poly(vinylidene fluoride) (PVDF)-based polymer electrolytes are particularly interesting due to their high ionic conductivity, high mechanical strength, excellent electrochemical stability, and good processability. However, some fundamental processes in these PVDF-based electrolytes, such as the ion-transport mechanism, are still not well understood.
Liangliang Li, Ce-Wen Nan, Tsinghua University, Beijing, China, and colleagues have investigated the influence of residual N,N-dimethylformamide (DMF) solvent on the performance of the PVDF-based electrolytes. The team prepared electrolyte membranes from PVDF and LiN(SO2F)2 (LiFSI) via a solution-casting method. The amount of residual DMF in the electrolytes was determined by gas chromatography (GC) and NMR spectroscopy.
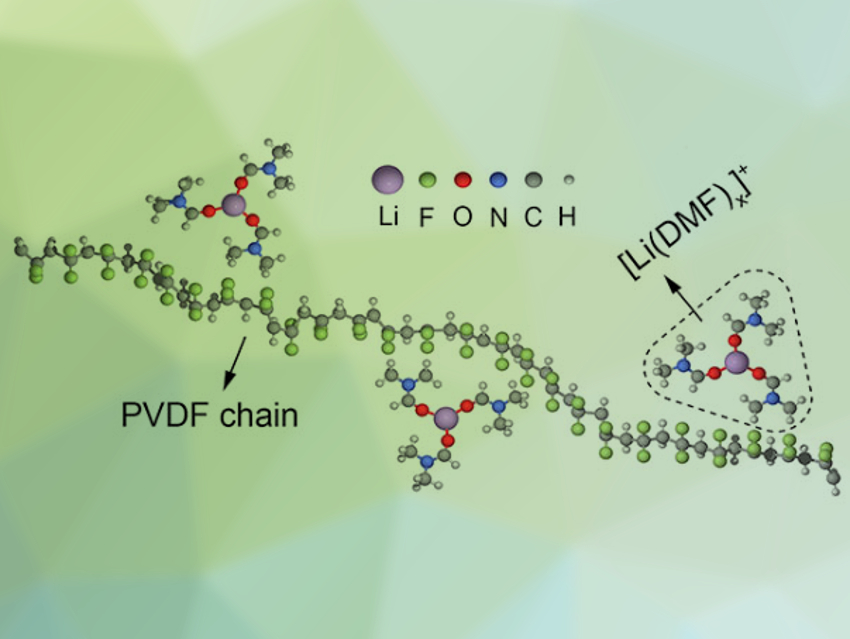
The team found that the activation barrier for lithium-ion transport is lower in electrolytes with a high DMF content and vice versa. The residual DMF in the electrolytes interacts strongly with lithium salts, which the researchers showed using infrared (IR) spectroscopy. The DMF molecules are complexed with Li+ ions to form [Li(DMF)x]+ (pictured above). The electrochemical stability of the PVDF-based electrolytes was found to be improved with a lower DMF content and vice versa.
The researchers then optimized the ionic conductivity and stability of the electrolyte by regulating the solvation effect and finding a compromise between these two opposing effects. Cells with a medium content of residual DMF in the electrolyte showed the best cycling performance over 1,000 cycles, with a capacity retention of 94 % at 0.35 C.
- High Cycling Stability for Solid‐State Li Metal Batteries via Regulating Solvation Effect in Poly(Vinylidene Fluoride)‐Based Electrolytes,
Xue Zhang, Jian Han, Xiangfu Niu, Chengzhou Xin, Chuanjiao Xue, Shuo Wang, Yang Shen, Liang Zhang, Liangliang Li, Ce‐Wen Nan,
Batteries Supercaps 2020.
https://doi.org/10.1002/batt.202000081