Multiple bonds are common in organic compounds featuring boron, carbon, nitrogen, and oxygen. The heavier relatives of these elements form weaker multiple bonds, which makes multiply bonded species difficult to isolate. Group 13/15 multiple bonds share the same number of outer-shell electrons as carbon–carbon bonds, however, they have significantly different reactivities.
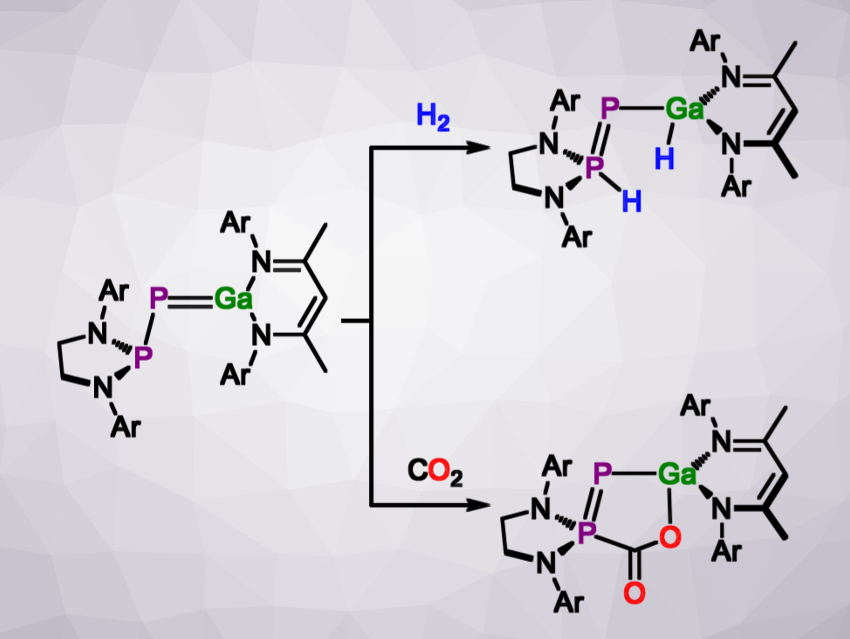
In their search for examples of group 13/15 multiple bonds of the heavier elements, Jose Goicoechea and colleagues, University of Oxford, UK, have obtained a species with a gallium–phosphorus double bond (pictured above on the left). The team used the tendency of phosphaketenes (R–P=C=O) to react with Lewis bases to liberate carbon monoxide. They used the gallium(I) carbene analogue (NacNac)Ga (NacNac = {(Me)C[(Dipp)N]}2, Dipp = 2,6-diisopropylphenyl) as the Lewis base, and reacted it with either the unsaturated [(HC)2(NDipp)2P]P=C=O or the saturated [(H2C)2(NDipp)2P]P=C=O in deuterated benzene. The phosphagallene products were characterized using NMR spectroscopy and X-ray crystallography.
The stability of the products depends on the saturation of the phosphanyl moiety. The product with an unsaturated phosphanyl ring rearranges in solution, while the saturated variant is stable even at elevated temperatures. When treated with hydrogen gas, this more stable phosphagallene undergoes a 1,3-addition across the phosphanyl and the gallium centers, resembling a frustrated Lewis pair in reactivity. Carbon dioxide can be captured in a similar manner (both reactions pictured above). Similar compounds may ultimately be used in catalytic reactions such as CO2 activation.
- A phosphanyl‐phosphagallene that functions as a frustrated Lewis pair,
Daniel W. N. Wison, Joey Feld, Jose Manuel Goicoechea,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202008207