Zeolites are microporous aluminosilicate minerals. They are often used as adsorbents or catalysts. Zeolites can provide unique activity via shape-selective catalysis, where the product formation depends on how the processed molecules fit in the material’s micropores near the active sites. While this concept has been used by the chemical industry for cost-effective processes since the 1960s, the fundamental understanding of the processes has remained rather limited so far.
Bert M. Weckhuysen and colleagues, Utrecht University, The Netherlands, have prepared anisotropic zeolite ZSM-5 crystals, in which different channel orientations dominate the outer surface of the crystals. The differently oriented ZSM-5 crystals were prepared using either trimer-tetrapropylammonium cations (trimer-TPA+) or tetrapropylammonium cations (TPA+) as structure-directing agents. The resulting anisotropic crystals allowed the researchers to study the formation of reactive intermediates in the different zeolite channels.
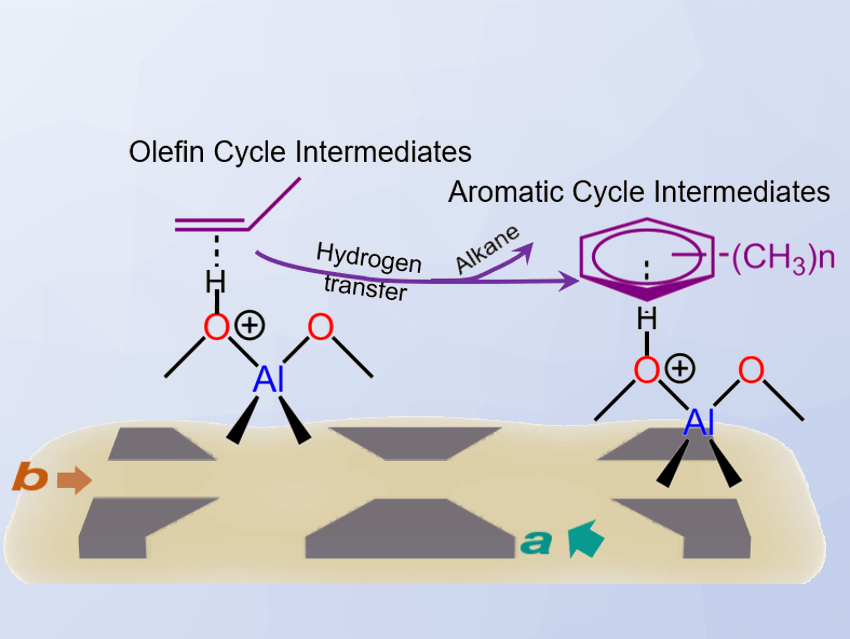
The team examined the relationship between these zeolite channels and the reaction intermediates formed during a methanol-to-hydrocarbons (MTH) process. For this, they used solid-state nuclear magnetic resonance (NMR) spectroscopy. The MTH process converts methanol into a range of products from olefins to gasolines. It features two inter-dependent reaction cycles, referred to as the aromatic cycle and the olefin cycle.
The researchers found that more constrained, curved channels favor smaller reactive intermediates, which produce higher alkenes via the olefin cycle (pictured left). Slightly more spacious straight channels favor more bulky reactive intermediates to produce ethylene via the aromatic cycle (pictured right). These insights could be useful for the development of zeolite materials that provide optimized yields of the desired products.
- Elucidating Zeolite Channel Geometry–Reaction Intermediates Relationships during the Methanol-to-Hydrocarbons Process,
Donglong Fu, Alessandra Lucini Paioni, Cheng Lian, Onno van der Heijden, Marc Baldus, Bert Marc Weckhuysen,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202009139