Cyclodextrins (CDs) are cyclic oligosaccharides commonly used hosts in host-guest chemistry. They have high chemical stability and can bind a wide variety of guest molecules in their cavities. The chemical modification of CDs can allow researchers to improve their inclusion ability toward guest molecules. However, their applications as hosts are usually limited to aqueous solutions and certain polar organic solvents, because the driving forces of the guest inclusion are mainly hydrophobic and van der Waals interactions.
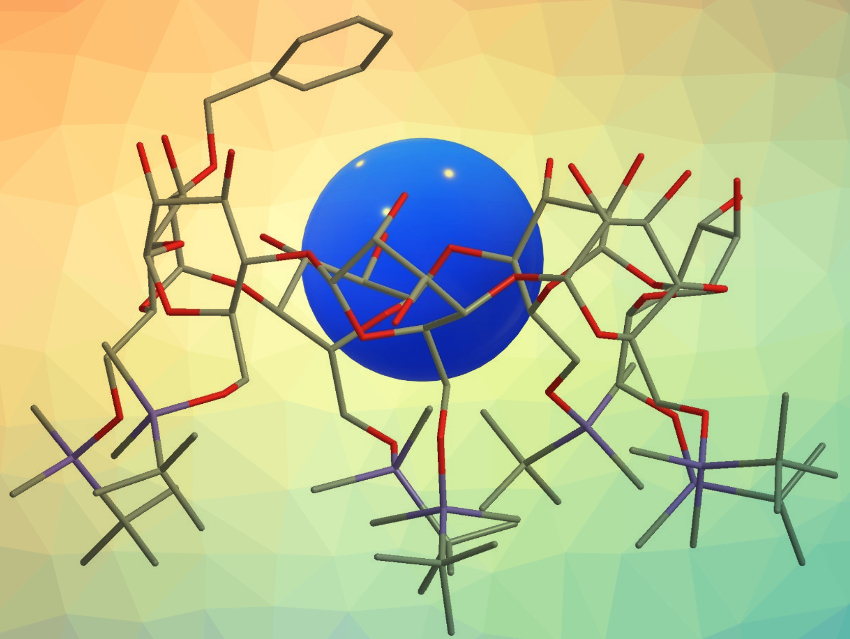
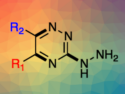
Toshiyuki Kida and colleagues, Osaka University, Japan, have designed and synthesized 6-O–tert-butyldimethylsilylated β-cyclodextrin (TBDMS-β-CD) derivatives with various aromatic substituents at the 2-O position. This functionalization enhances the guest inclusion in organic solvents through cooperative guest binding between the CD cavity and the aromatic substituent. The team modified the 2‐OH group of TBDMS‐β‐CD via reactions with benzyl bromide, 1‐(bromomethyl)‐3‐methylbenzene, 1‐(bromomethyl)‐4‐methylbenzene, 2‐(chloromethyl)pyridine hydrochloride, or 3‐(chloromethyl)pyridine hydrochloride in the presence of sodium hydride in tetrahydrofuran (THF).
Compared to the nonmodified TBDMS-β-CD, functionalized TBDMS-β-CDs with m– and p-tolyl substituents at the 2-O position exhibit a 100-fold increase in inclusion ability toward pyrene in nonpolar solvents. Certain aromatic substituents, i.e., p‐tolyl and 2‐picolyl, can also enhance the chiral recognition ability of TBDMS-β-CD toward chiral naphthalene derivatives. The modified CDs have potential uses as catalysts for enantioselective reactions in organic solvents and as effective adsorbents for chiral chromatography.
- Control of Guest Inclusion and Chiral Recognition Ability of 6‐O‐Modified β‐Cyclodextrins in Organic Solvents by Aromatic Substituents at the 2‐O Position,
Justine M. Kalaw, Ryusuke Yamamoto, Chizuru Kogame‐Asahara, Hajime Shigemitsu, Toshiyuki Kida,
ChemPlusChem 2020.
https://doi.org/10.1002/cplu.202000522