Value-Added Chemicals from CO2
Carbon dioxide is not just an undesirable greenhouse gas, it is also an interesting source of raw materials that are valuable and can be recycled sustainably. Arjan W. Kleij, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and Catalan Institute of Research and Advanced Studies (ICREA), Barcelona, Spain, and colleagues have developed a new catalytic process for converting CO2 into valuable chemical intermediates in the form of cyclic carbonates.
Getting CO2 to react is, unfortunately, not easy. Currently, most research is focused on the conversion of CO2 into methanol, which can be used as an alternative fuel as well as a feedstock for the chemical industry. Innovative catalytic processes could allow CO2 to be converted into valuable chemical compounds without taking a detour through methanol, perhaps for the production of biodegradable plastics or pharmaceutical intermediates.
Organic Carbonates
One highly promising approach is the conversion of CO2 into organic carbonates. The researchers have developed a conceptually new process to produce carbonates in the form of six-membered rings, starting from CO2 and basic, easily accessible building blocks. These cyclic carbonates have great potential for the creation of new CO2-based polycarbonates.
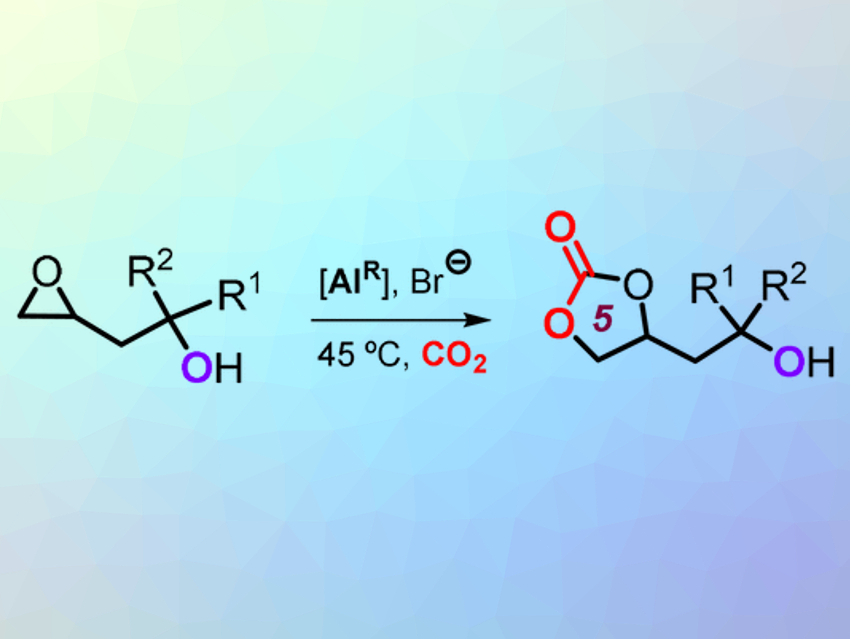
The starting materials are compounds with a carbon–carbon double bond and an alcohol group (–OH) on a neighboring carbon atom (homoallylic alcohols). In the first step of the reaction, the double bond is converted into an epoxide. The epoxide can react with CO2 in the presence of a specific catalyst (reaction pictured). The product is a cyclic carbonate in the form of a five-membered ring.
Kinetically Controlled Cascade Process
In the next step, an organic catalyst (N-heterocyclic base) activates the OH group and causes the five-membered ring to rearrange into a six-membered ring. The oxygen atom from the OH group is integrated into the new ring, while one of the oxygen atoms from the original five-membered ring forms a new OH group. However, the reverse reaction also takes place because the original five-membered ring is significantly more energetically favorable, and only a vanishingly small amount of the six-membered ring is present at equilibrium. The trick is to trap the six-membered ring. The new OH group binds to a reagent (acylation) because its different position makes it considerably more reactive than the original OH group.
This newly developed process gives access to a broad palette of new six-membered carbonate rings in excellent yields, with high selectivity and under mild reaction conditions. This widens the repertoire of CO2-based heterocycles and polymers, which are difficult to produce by conventional methods.
- Organocatalytic Trapping of Elusive Carbon Dioxide Based Heterocycles by a Kinetically Controlled Cascade Process,
Chang Qiao, Alba Villar‐Yanez, Josefine Sprachmann, Bart Limburg, Carles Bo, Arjan W. Kleij,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202007350