Isoquinolinones, a class of aromatic N-heterocycles, are often found in natural products and bioactive molecules. Straightforward synthetic access to functionalized isoquinolin-1(2H)-ones is, thus, useful. Different functional groups have been introduced into the C3/C4/C8 positions using transition-metal-catalyzed C–H functionalizations.
Guo-Li Huang, Yunnan Normal University, Kunming, China, and colleagues have developed a metal-free, green, simple, and efficient iodine-promoted method for the synthesis of 4-arylthioisoquinolin-1(2H)-ones under solvent-free conditions. The team used inexpensive and commercially available aryl sulfonyl chlorides as the sulfur source. Iodine (I2) and diethyl phosphite ((EtO)2P(O)H) allow the generation of electrophilic ArSI species, which can be converted to ArS· radical intermediates by homolysis. These intermediates can couple with the substrate and form the desired products.
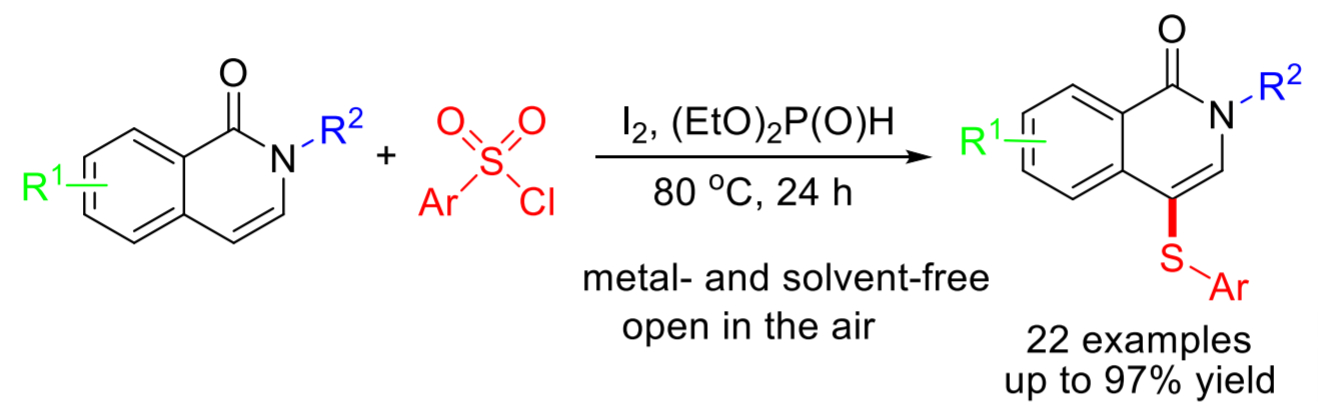
The reaction proceeds smoothly in air and gives moderate to good yields of the C-4 sulfenylated products. The process has high regioselectivity, a broad substrate scope, and good functional group tolerance. Overall, the method provides simple, time-efficient, scalable, and low-cost access to 4-arylthio-substituted isoquinolin-1(2H)-one derivatives.
- I2-promoted direct C-H sulfenylation of isoquinolin-1(2H)-ones with sulfonyl chlorides,
Cai-Yun Yang, Xia Li, Bo Liu, Guo-Li Huang,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202001371

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

