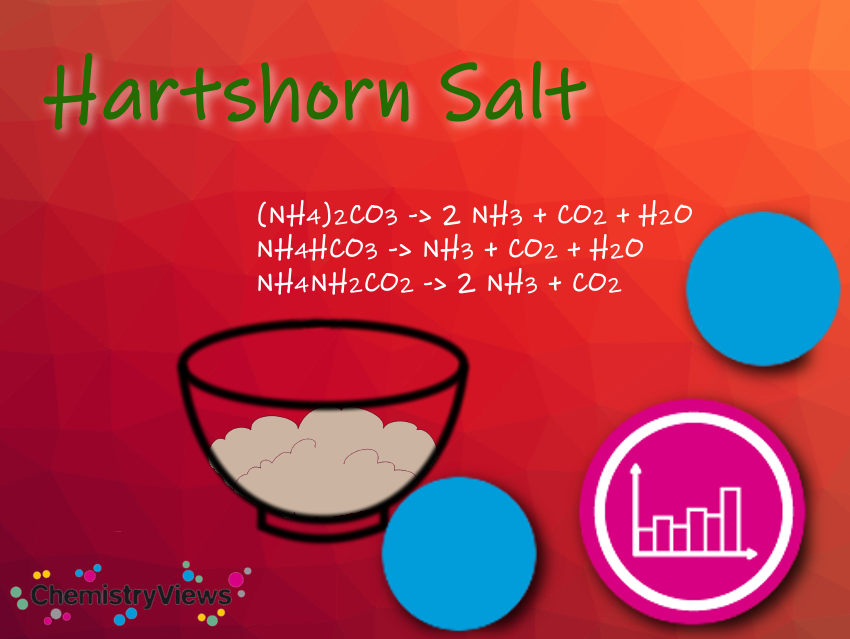
Hartshorn salt, also known as hartshorn, or baker’s ammonia, used to be obtained from horn substance such as hooves or antlers. It is a mixture of two parts ammonium hydrogen carbonate (NH4HCO3) and one part ammonium carbonate ((NH4)2CO3) as well as some ammonium carbamate (NH4CO2NH2). It is a traditional leavening agent for baking gingerbread. Since it strongly promotes acrylamide formation, full or partial replacement with baking powder or baking soda is recommended (see belowunder Health Advice).
.png)
In gingerbread doughs, hartshorn salt is often used together with potash. The combination of potash and hartshorn salt prevents the dough from spreading too widely during baking. Potash is crucial for a wide, flat pore, while hartshorn salt ensures a large-pored, upward shoot in the pastry. When potash is used together with hartshorn salt for dough leavening, both substances are dissolved separately and added to the dough one after the other. If hartshorn salt is dissolved together with potash and added to the dough, both substances react with each other and lose their leavening power.
Health Advice
For flat, dry pastries, ammonia, like carbon dioxide, escapes through the air, so there is nothing wrong with occasional consumption of pastries containing hartshorn salt from this point of view. However, hartshorn salt is unsuitable as a leavening agent for higher, moist types of pastry, since the ammonia will be trapped and it may not be able to escape completely and reacts with water to form an ammonia solution. This is a health hazard. It discolors the crumb greenish, and the pastry will get an unpleasant taste.
Baked goods with hartshorn salt have higher levels of acrylamide. Ammonia released during the baking process reacts with glucose and fructose to form intermediates that in turn, react with asparagine, an amino acid found in nuts, seeds, and whole grains to form acrylamide. Acrylamide is found in many carbohydrate-rich foods that have been heavily heated and have a low water content. It potentially increases the risk of cancer in humans. In the adult diet, coffee and fried or deep-fried potato products are the largest sources of acrylamide, followed by cookies, crackers, crispbread, and toast.
References
- Holleman Wiberg, Lehrbuch der Anorganischen Chemie, de Gruyter, Berlin, Germany 1985.
- G. Schwedt, Noch mehr Experimente mit Supermarktprodukten, Wiley-VCH, Weinheim, Germany 2003.
- Andrea Beckmann, Janina Langner, Backpulver – Warum der Kuchen aufgeht?, Chemie im Haushalt, Westfälische Wilhelms-Universität Münster, 2007.
- Ruth Genuneit, Gewürze und Backtriebmittel in der Weihnachtsbäckerei, Institut Dr. Flad, Stuttgart, Germany, 2019. (Retrieved November 24, 2020)
Also of Interest
- Chemistry Advent Calendar 2020
ChemistryViews 2020.
Daily highlights from the chemistry of spices - Potash Chemistry
ChemistryViews 2020.11280254 - Baking Powder Chemistry
ChemistryViews 2020.