Some useful drugs consist of peptides acting on their protein targets. To make them more efficient and stable, Gilles Guichard, University of Bordeaux, Institut Européen de Chimie et Biologie, Pessac, France, and colleagues have found a way to replace crucial segments of the peptides with ureido units. These oligoureas, which are composed of urea-based units, fold into a structure similar to that of peptides. Oligourea-based “fake” peptides enhance the options for rational drug design.
Peptide Mimics
Several drugs are peptides that inhibit or activate the actions of certain proteins. To enhance their efficiency, scientists are investigating peptide mimics. Peptide mimics contain strands of small organic units that resemble amino acids—the building blocks of peptides—but are not identical to them. The rationale is that proteolytic enzymes are less likely to attack such fake peptide strands, so the drugs would be more effective.
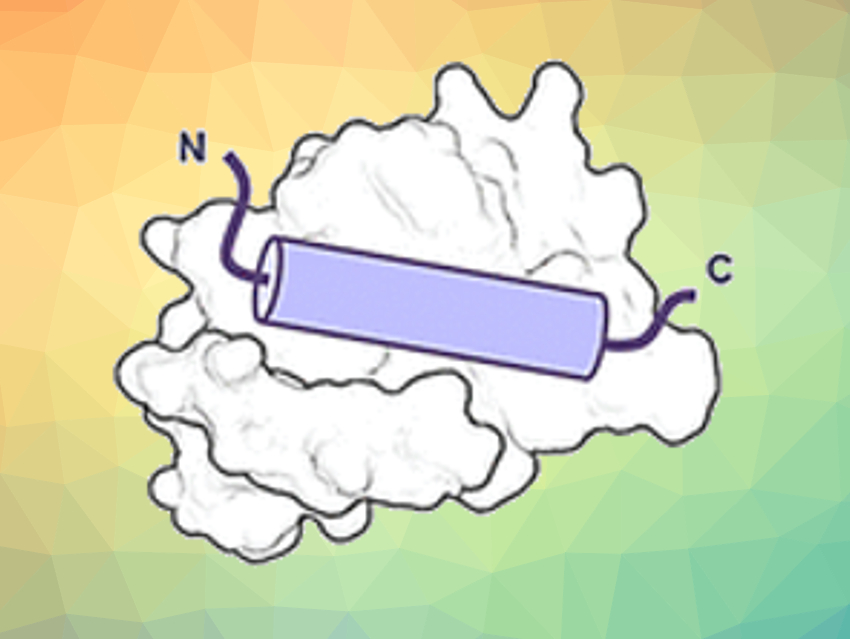
However, the synthetic strands—called oligomers—must fold into the structure of the original peptide to bind to its target protein properly. The team has explored oligomers made of ureido units, which are derivatives of urea. These oligoureas fold into a helix, one of the hallmark structures of peptides. However, there are slight differences. “Oligourea helices have fewer residues per turn, a smaller rise per turn, and a larger diameter than the original peptide α-helix,” says Guichard.
Oligourea Foldamers
To determine whether oligoureas could mimic real peptide structures, the researchers prepared peptide–oligourea hybrids and investigated their binding to target proteins. One of the targets, MDM2, is a natural regulator of the tumor suppressor protein p53. The other one, VDR, is a protein required in the regulation of cell growth, immunity, and other biological functions.
For the MDM2-binding peptide mimic, the researchers prepared hybrids by replacing three terminal key amino acids with ureido units. For the VDR-binding peptide mimic, they replaced the middle amino acid segment with ureido units. After some rounds of optimization, the team found hybrid structures with high binding affinities.
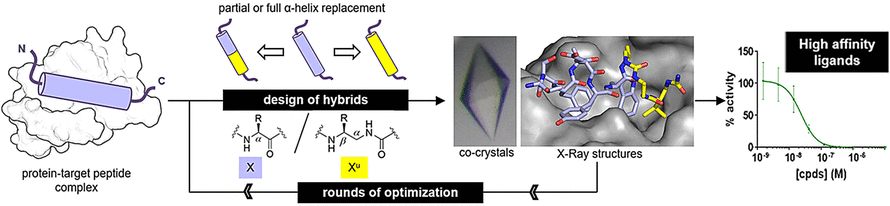
The binding affinities were similar to those of the original peptides. X-ray analysis revealed that the hybrid structures adopted a regular helical structure. However, the helices were still a bit wider and had larger spaces between the side chains along the oligourea backbone than those of natural peptides.
Peptide–oligourea hybrids are expected to resist proteolytic degradation, an important goal in medicinal chemistry. Another advantage is that they allow more modifications. “α-Amino acids can be substituted at two positions, but ureido units have one site more,” says Guichard. This means that hybrid peptide–oligourea drugs offer more options for optimization.
- Structural Basis for α‐Helix Mimicry and Inhibition of Protein–Protein Interactions with Oligourea Foldamers,
Léonie Cussol, Laura Mauran‐Ambrosino, Jérémie Buratto, Anna Y. Belorusova, Maxime Neuville, Judit Osz, Sébastien Fribourg, Juliette Fremaux, Christel Dolain, Sébastien R. Goudreau, Natacha Rochel, Gilles Guichard,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202008992