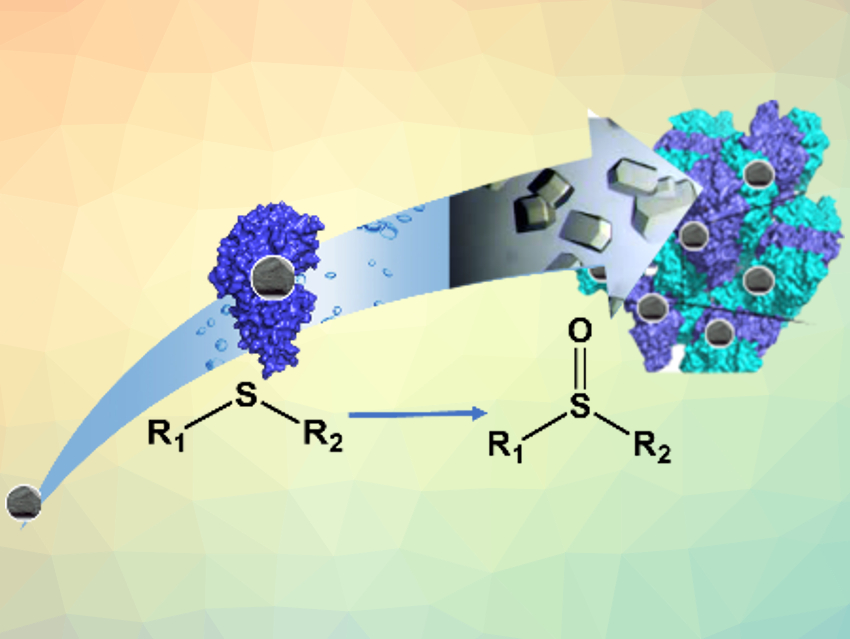
Biocatalysis can be useful for green, sustainable processes in chemistry. However, it often has drawbacks compared with metal‐based chemical catalysis. Heterogeneous inorganic catalysts, for example, can provide better stability and recyclability than natural enzymes. One way to address these drawbacks is engineering natural enzymes and turning them into biohybrids, in which a protein hosts an “unnatural” active site—usually an inorganic catalyst. Immobilizing the resulting hybrid enzymes by crosslinking them to give so-called crosslinked enzyme crystals (CLEC) can stabilize them and make recovery and reuse easier.
Stéphane Ménage and colleagues, Université Grenoble Alpes, France, have designed an artificial biohybrid enzyme for thioether sulfoxidation (reaction pictured). The team incorporated iron complexes with nitrogen‐based ligands into the nickel-binding protein NikA. The resulting proteins were crosslinked to give CLEC using a glutaraldehyde solution. This leads to a hybrid catalyst in which the iron-based complex plays the role of the active site and the protein host drives the selectivity of the reaction. The CLEC approach makes the hybrid catalysts highly robust.
The resulting crystals can catalyze thioether sulfoxidation efficiently. They provide improved stability, higher yields, faster kinetics, and a wider substrate scope than their counterpart in solution. According to the researchers, crystalline artificial enzymes could be useful alternatives to soluble or supported enzymes.
- A Selective Sulfide Oxidation Catalyzed by Heterogeneous Artificial Metalloenzymes Iron@NikA,
Sarah Lopez, Caroline Marchi‐Delapierre, Christine Cavazza, Stéphane Ménage,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202003746