Natural heme-binding proteins use an iron-porphyrin cofactor to catalyze the disproportionation of H2O2, the oxidation and haloperoxidation of organic substrates, and oxygenation reactions—all related to fundamental biological processes. The protein environment and variations in the heme coordination play important roles in defining these different reactivities. Man-made synthetic heme proteins can be used to investigate these effects and to produce efficient green catalysts for pharmaceutical and industrial applications.
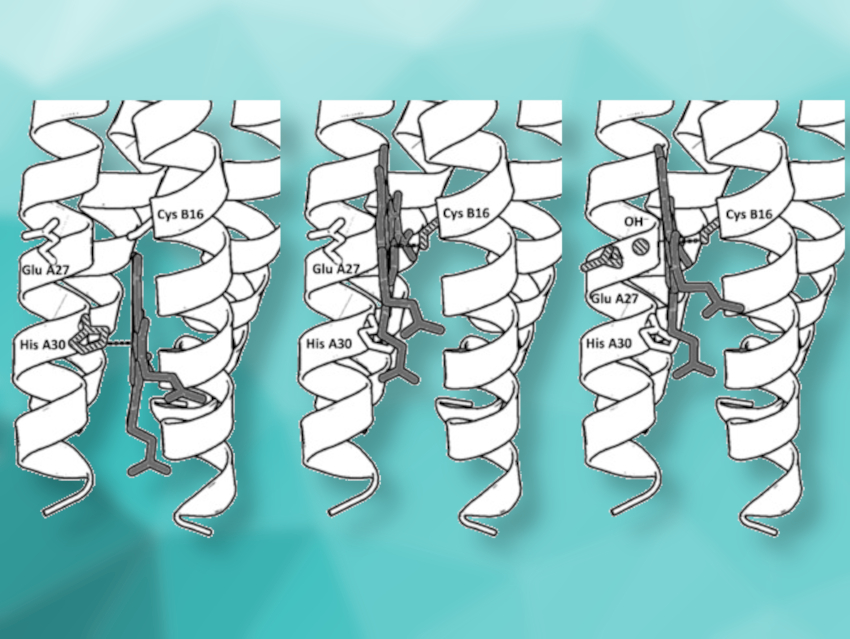
Anabella Ivancich, Centre National de la Recherche Scientifique (CNRS) and Aix-Marseille University, Marseille, France, Vincent L. Pecoraro, University of Michigan, Ann Arbor, USA, and colleagues have synthesized peptides that self‐assemble into α‐helical structures and provide a switchable coordination environment for the heme cofactor. The researchers found a pH-dependent switch in the heme-binding mode within this single synthetic protein. Using spectroscopic studies, they found three coordination modes: histidine‐pentacoordinated heme at pH 7 (pictured above on the left), cysteine‐pentacoordinated heme at pH 9.0 (pictured above in the center), and cysteine/hydroxide hexacoordinated heme at pH 10.5 (pictured above on the right).
The resulting miniature protein can catalyze O2 reduction (like cytochrome P450 monooxygenases) as well as substrate oxidation (like peroxidases). Thus, the designed α‐helical scaffold could be useful to explore heme enzyme chemistry.
- The pH‐Induced Selectivity Between Cysteine or Histidine Coordinated Heme in an Artificial α‐Helical Metalloprotein,
Karl J. Koebke, Toni Kühl, Elisabeth Lojou, Borries Demeler, Barbara Schoepp‐Cothenet, Olga Iranzo, Vincent L. Pecoraro, Anabella Ivancich,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202012673