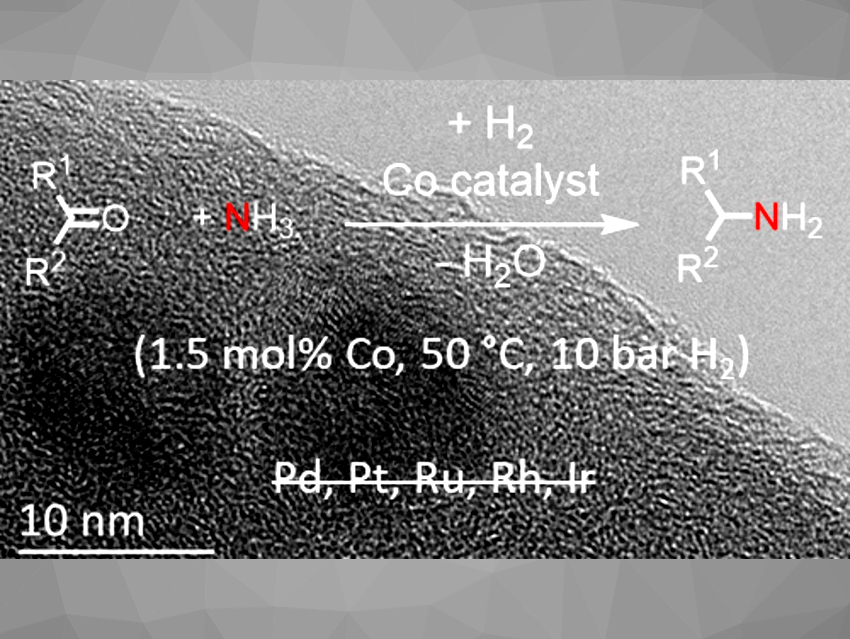
C–N bond-formation reactions are important in organic synthesis. Amines are key functional groups in many bulk and fine chemicals, pharmaceuticals, and materials. The synthesis of primary amines can be particularly challenging. Catalytic reductive aminations that use hydrogen as a green reducing agent are attractive reactions for the synthesis of amines. Using catalysts based on earth-abundant metals instead of rare noble metals, such as Pd, Pt, Rh, or Ir for such reactions would be preferable. Reusable nanostructured cobalt catalysts, for example, can be used for the synthesis of primary amines. However, due to the low activity of these nanostructured 3d metal catalysts compared with noble metals, harsh reaction conditions are required.
Rhett Kempe, University of Bayreuth, Germany, and colleagues have developed a higly active cobalt catalyst for the synthesis of primary amines via reductive amination, using hydrogen as the reducing agent and easy-to-handle aqueous ammonia as the nitrogen source. The team first prepared a catalyst support by crosslinking the polycarbosilane precursor SMP 10 and acrylonitrile in dimethylformamide using azobisisobutyronitrile as an initiator, followed by pyrolysis and an NaOH treatment to give an N-doped amorphous carbon material. The support was then impregnated with an aqueous solution of Co(NO3)2·6 H2O dissolved in water, pyrolyzed under N2, and reduced under an N2/H2 mixture.
The resulting catalyst operates under mild conditions (50 °C, 10 bar H2) with a catalyst loading of 1.5 mol%, outperforms commercially available noble metal catalysts, and can be reused. The amination reaction (pictured) has a broad substrate scope and good functional group tolerance. The team attributes the high activity of the catalyst to the amorphous N-doped carbon support, which has small graphitic domains and pores that carry basic NH-functionalities. These NH groups can bind cobalt species and might be involved in catalyzing the condensation step.
- Co-Catalyzed Synthesis of Primary Amines via Reductive Amination Employing Hydrogen under Very Mild Conditions ‐ A Nanostructured 3d Metal Catalyst Outperforms Noble Metal Catalysts,
Rhett Kempe, Matthias Elfinger, Timon Schönauer, Sabrina Thomä, Robert Stäglich, Mirijam Zobel, Jürgen Senker, Markus Drechsler,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202100553