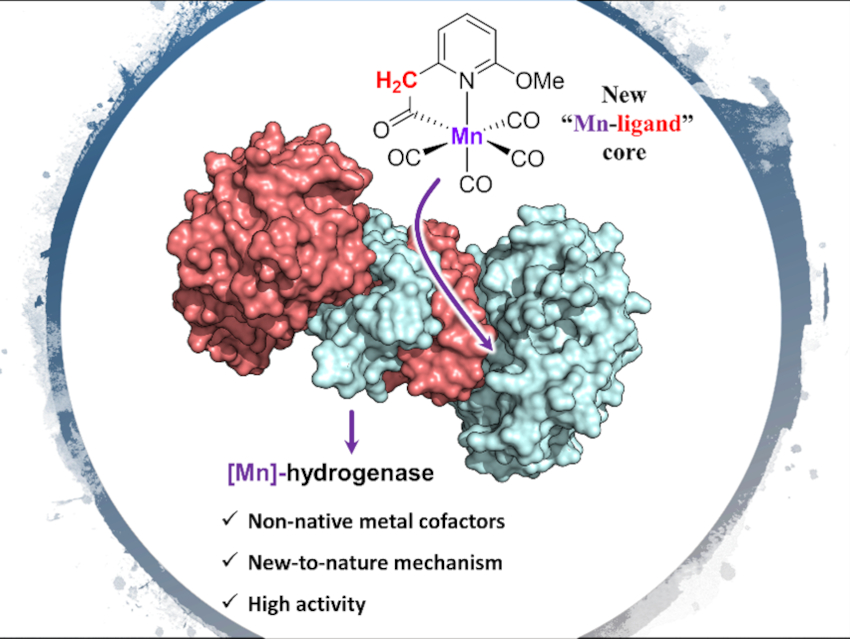
Artificial enzymes that incorporate non-native metal cofactors while showing native enzyme activities are uncommon. However, such artificial enzymes could be valuable alternatives for hard-to-access enzymes, important tools for mechanistic studies, or useful catalysts for wider substrate scopes.
Xile Hu, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, Seigo Shima, Max Planck Institute for Terrestrial Microbiology, Marburg, Germany, and colleagues have designed and synthesized a series of Mn complexes (pictured below) as mimics of the active site of [Fe]-hydrogenase. The team prepared the complexes starting from pyridine derivatives, which were deprotonated by n‐BuLi to give a lithiated salt and then treated with Mn(CO)5Br to give the desired Mn complexes. The reconstitution of these complexes with the apo-enzyme (an enzyme without its cofactor) of [Fe]-hydrogenase then gave semi-synthetic [Mn]-hydrogenases.
.jpg)
The researchers measured enzymatic activities for the native hydrogenation and dehydrogenation reactions photometrically. Most of the reconstituted enzymes with the wild-type apo-enzyme showed catalytic activity. The activity of the complexes and the [Mn]-hydrogenases confirmed the essential role of a metal-ligand cooperative site in the Mn complexes: A suitable internal base in the Mn complex is required for reconstituting an active [Mn]‐hydrogenase.
The team also found that, due to the nature and position of the internal base, the mode of metal-ligand cooperation in two of the active [Mn]-hydrogenases is different from that of the native [Fe]-hydrogenase. One of the prepared [Mn]-hydrogenases shows the highest known specific activity for semi-synthetic [Mn]- and [Fe]-hydrogenases so far.
- Diversifying metal-ligand cooperative catalysis in semi-synthetic [Mn]-hydrogenases,
Huijie Pan, Gangfeng Huang, Matthew Wodrich, Farzaneh Fadaei Tirani, Kenichi Ataka, Seigo Shima, Xile Hu,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202100443