Can batteries be fast-charging/discharging as well as non-explosive? Transition-metal metallocenes have a rich redox chemistry and can allow fast electron transfer. This makes them interesting candidates for use in batteries. For example, ferrocene- and cobaltocenium-based organic redox batteries (ORBs) have potential as non-toxic, high-energy, and high-power-density alternatives to lithium-based batteries. However, they have not reached commercialization yet.
Seyyed Mohsen Beladi-Mousavi, Lorenz Walder, University of Osnabrück, Germany, and colleagues have prepared an all-metallocene rechargeable battery. This ORB consists of a poly-ferrocenium-based cathode and a poly-cobaltocene-based anode. Both of these redox polymers were embedded in porous graphene via self-assembly. The resulting polymer@GO materials were dropcast onto current collectors to create poly-metallocene-based films on the electrodes. Finally, the GO was transformed to reduced GO (rGO) electrochemically.
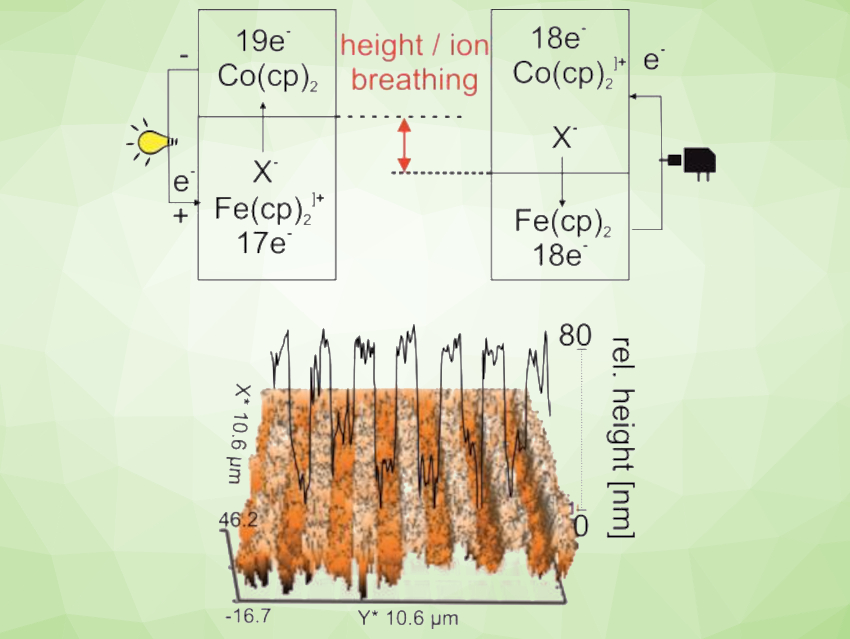
The resulting battery’s fast charging and discharging is due to the ultrafast electron transfer between the metallocene centers, the high electronic conductivity of graphene, and the porous structure of the metallocene composite. The non-explosive property of the battery results from the use of an aqueous LiClO4 electrolyte and from a phenomenon called “harmonic height breathing”. Height breathing is the increase or decrease of the height of the active battery materials when ions flow in or out during (dis)charging. This has long been considered problematic for ORBs. However, according to the researchers, no pressure builds up in the closed organic metallocene battery because the individual height movements within the cell are reciprocal and formally cancel each other out.
- The Metallocene Battery ‐ Ultrafast Electron Transfer Self Exchange Rate Accompanied by a Harmonic Height Breathing,
Seyyed Mohsen Beladi-Mousavi, Shamaila Sadaf, Ann-Kristin Hennecke, Jonas Klein, Arsalan Mado Mahmood, Christian Rüttiger, Markus Gallei, Fangyu Fu, Jaime Ruiz, Didier Astruc, Lorenz Walder,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202100174