In a number of biological processes, iron–sulfur clusters play a vital role, acting as cofactors to enzymes. Daniel L. M. Suess, Massachusetts Institute of Technology (MIT), Cambridge, MA, USA, and colleagues have found that cubic clusters can support unusual bonding states. The work shows that the cluster copes well with a multiple bond between iron and nitrogen—a structural motif that may be involved in biological nitrogen fixation.
Cube-Shaped Iron–Sulfur Clusters
Clusters made of iron and sulfur atoms are essential cofactors for a number of enzymes, especially in biological processes involving electron transfer. As an example, nitrogen-fixing bacteria use iron–sulfur clusters to convert nitrogen from the air into useful nitrogen compounds. To understand this important biological process, scientists dig deep into the bonding relationships possible between nitrogen and iron atoms in such clusters.
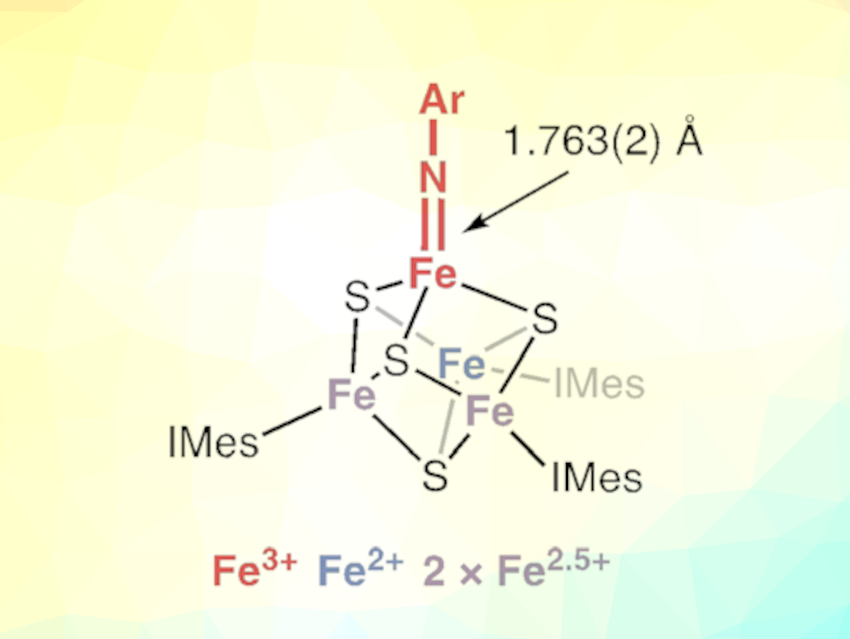
The researchers investigated the cluster’s capability to form unusual bonds between iron and nitrogen. A double bond, as part of an imide, may play a role in nitrogen fixation. To construct the imide, the team began by producing a cube-shaped iron–sulfur cluster. The eight corners of the cube are occupied by alternating iron and sulfur atoms; three of the iron atoms are protected by ligands. The remaining unshielded iron atom of the cluster was bound to a replaceable chloride ligand. Careful selection of the reagents enabled the team to swap out the chloride ion and then, by oxidation with a nitrogen-containing reagent, the tricky double bond between the unique iron atom and the nitrogen atom—and thus the imide group—was formed (pictured).
Multiple Bond between Iron and Nitrogen
The researchers expected that the iron–nitrogen double bond could strongly distort the cluster’s structure. Instead, to their surprise, they observed only minor structural changes. The team’s spectroscopic studies explain this finding: the electron-rich imide pushes away electron density from the neighboring sulfur and iron atoms, and the totality of these minor effects is what allows the cluster to accommodate the imide bond. “These findings demonstrate a dynamic interplay between iron–nitrogen, iron–sulfur, and iron–iron bonding,” state the researchers.
The new imido-bound cluster was able to cleave weak carbon–hydrogen bonds from organic reagents. The researchers intend to use these studies as a starting point for further investigations of the reactivity of imide-bound iron–sulfur clusters. “This highlights the promise of exploiting the synergy between the structural robustness and electronic flexibility of these fundamental cofactors,” Suess says.
- A Terminal Imido Complex of an Iron–Sulfur Cluster,
Arun Sridharan, Alexandra C. Brown, Daniel L. M. Suess,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202102603