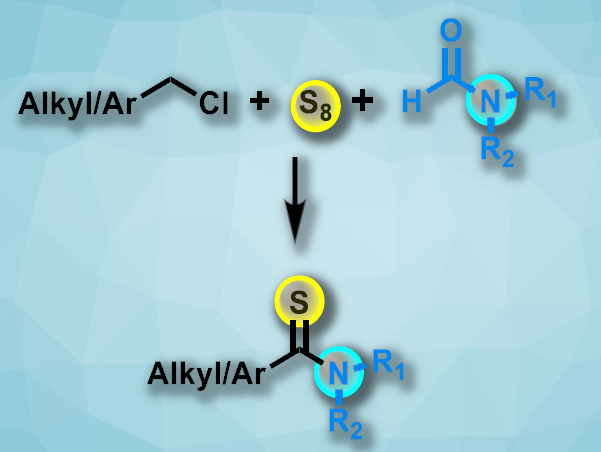
Thioamides are found, e.g., in pharmaceutically active compounds. They can also be useful in the synthesis of sulfur-containing heterocycles. Existing methods for the preparation of thioamides often require harsh conditions, long reaction times, and/or toxic reagents. Elemental sulfur could be an environmentally friendly reagent for the synthesis of thoamides. However, sulfur can poison metal-based catalysts, and known metal-free approaches have limited substrate scopes.
Shaodong Zhou, Zhejiang University, Hangzhou, China, Xin Ge, Jiangnan University, Wuxi, China, and colleagues have developed a transition-metal-free method for the one-pot synthesis of thioamides via a three-component reaction involving chlorohydrocarbons, amides, and elemental sulfur (pictured). The team reacted a variety of alkyl or aryl chlorides with amides such as N,N-dimethylformamide, N-methylformamide, N-formylmorpholine, or N-formylpiperidine in the presence of elemental sulfur and NaOH. The reactions were performed under an air atmosphere at 100 °C.
Both alkyl and aryl thioamides were obtained in moderate to good yields with a good functional group tolerance. The strategy avoids residual transition metal in the product and does not require additional oxidants.
- Transition‐Metal‐Free, General Construction of Thioamides from Chlorohydrocarbon, Amide and Elemental Sulfur,
Hao Jin, Xinzhi Chen, Chao Qian, Xin Ge, Shaodong Zhou,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100588
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)


