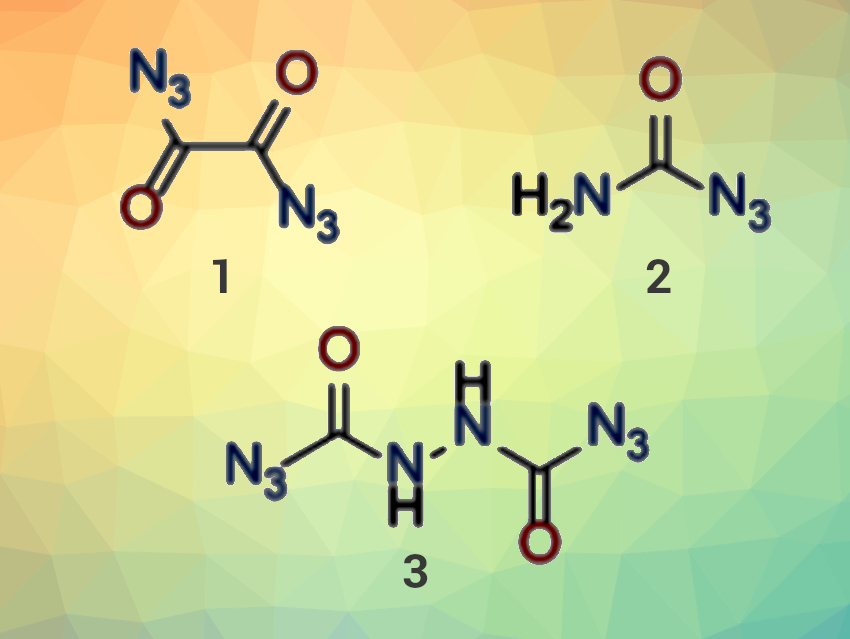
Carbonyl azides were first described more than a century ago by Curtius and Thiele in 1894 [1,2]. Today, carbonyl azides are used as intermediates in organic chemistry in the formation of reactive isocyanates and amines. However, as pure compounds, carbonyl azides (small ones, in particular) have a bad reputation due to their high explosive sensitivity and instability. Consequently, most reported examples have only been poorly characterized.
Thomas M. Klapötke, Ludwig Maximilian University (LMU) Munich, Germany, and colleagues have synthesized and characterized particularly small carbonyl azide derivatives such as oxalyl diazide (pictured, 1), carbamoyl azide (2), and N,N‘-bis(azidocarbonyl)hydrazine (3). The carbonyl azides were synthesized from hydrazino precursor compounds by careful diazotation at low temperatures. Carbamoyl azide could also be obtained from oxalyl diazide via a Curtius rearrangement to the reactive isocyanate, followed by a reaction with water. The team was also able to trap this isocyanate with hydroxy-functionalized or amino-functionalized molecules, i. e., methanol, oxetan-3-ol, and 2-amino-5H-tetrazole.
All synthesized compounds were investigated by NMR spectroscopy, infrared (IR) spectroscopy, and X-ray diffraction, and their thermal and physical stability were evaluated. Carbamoyl azide was found to be particularly stable toward external stimuli such as impact or friction due to the strong hydrogen bonds in the solid material.
- Synthesis, Characterization and Energetic Performance of Oxalyl diazide, Carbamoyl azide and N,N‘-bis(azidocarbonyl)hydrazine,
Thomas M. Klapötke, Alexander G. Harter, Jörg Stierstorfer, Michael Voggenreiter, Xiaoqing Zeng,
ChemPlusChem 2021.
https://doi.org/10.1002/cplu.202100214
References
- [1] Umlagerung von Säureaziden, R.CON3, in Derivate alkylirter Amine (Ersatz von Carboxyl durch Amid),
T. Curtius,
Ber. Dtsch. Chem. Ges. 1894, 27, 778–781.
https://doi.org/10.1002/cber.189402701152 - [2] Ueber Semicarbazid,
J. Thiele, O. Stange,
Liebigs Ann. Chem. 1894, 283, 1–46.
https://doi.org/10.1002/jlac.18942830102