The Friedel-Crafts alkylation is an important carbon–carbon bond formation reaction. It involves the alkylation of an aromatic compound with an alkyl halide in the presence of a strong Lewis acid. Enzyme catalysts could be an alternative to traditional Friedel-Crafts alkylation catalysts. They have advantages such as operation under mild reaction conditions, high selectivity, and the possibility of engineering the enzymes using directed evolution.
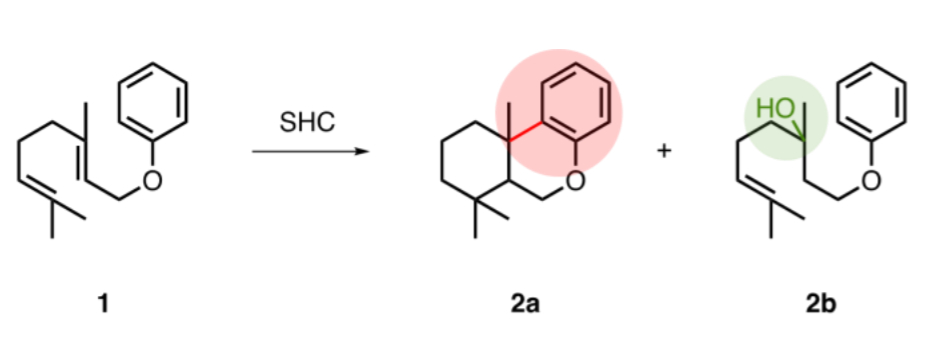
Bernhard Hauer, University of Stuttgart, Germany, and colleagues have found that the enzyme squalene-hopene cyclase (SHC) from Alicyclobacillus acidocaldarius and some of its variants can catalyze intramolecular Friedel-Crafts-type alkylations. The team used, e.g., geranyl phenylether (pictured below, 1) as a substrate and observed the formation of the Friedel-Crafts alkylation product (2a) as well as a hydration product (2b).
Using a library of SHC enzyme variants, the team demonstrated that minor changes in the enzyme can be used to generate more selective alkylation catalysts with significantly increased product formation. According to the researchers, this type of enzymatic catalyst could, for example, be useful for converting more diverse substrates as well as for intermolecular reactions in the future.
- Enzymatic Friedel‐Crafts Alkylation Using Squalene‐Hopene Cyclases,
Sabrina Henche, Bettina M. Nestl, Bernhard Hauer,
ChemCatChem 2021.
https://doi.org/10.1002/cctc.202100452